Correct Answer

verified
Correct Answer
verified
Short Answer
How many peaks appear in the proton spin decoupled 13C NMR spectrum of the compound below? 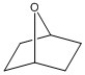
Correct Answer

verified
Correct Answer
verified
Short Answer
Deduce the identity of the following compound from the spectral data given.
C9H10O2: 13C NMR, δ 18.06 (quartet), 45.40 (doublet), 127.32 (doublet), 127.55 (doublet), 128.61 (doublet), 139.70 (singlet) (ppm), 180.98 (singlet); IR, broad 3500-2800, 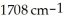
Correct Answer

verified
Correct Answer
verified
Essay
Predict the number of signals expected, their splitting, and their relative area in the 1H NMR spectrum of 2-methylpropane (isobutane).
Correct Answer

verified
2 signals:...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following reasons best explains why aromatic protons have a larger chemical shift than protons one carbon removed from a halogen?
A) An aromatic ring is more electron withdrawing than a halogen.
B) A halogen is more electron withdrawing than an aromatic ring.
C) Electron movement induces a magnetic field opposing the external field.
D) Electron movement induces a magnetic field reinforcing the external field.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The protons marked Ha and Hb in the molecule below are ________. 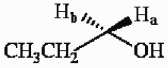
A) chemically equivalent
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy difference between the allowed spin states for an 1H nucleus is ________ the strength of the external magnetic field in which it is placed.
A) independent of
B) directly proportional to
C) inversely proportional to
D) exponentially related to
E) logarithmically related to
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of dibutyl ether.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What compound exhibits only two signals in its 1H NMR spectrum, a triplet and a quintet?
A) BrCH2CH2CH2Br
B) BrCH2CH2CH2Cl
C) (CH3) 2CHCH(CH3) 2
D) CH3CH2CH2CH3
E) (CH3) 2CHOCH(CH3) 2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many peaks appear in the proton spin decoupled 13 C NMR spectrum of the compound below? 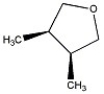
A) 2
B) 3
C) 4
D) 5
E) 6
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Deduce the identity of the following compound from the 1H NMR data given. C5H10O: δ 1.1 (6H, doublet), 2.2 (3H, singlet), 2.5 (1H, septet) (ppm)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The protons marked Ha and Hb in the molecule below are ________. 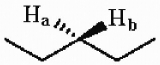
A) chemically equivalent or homotopic
B) enantiotopic
C) diastereotopic
D) endotopic
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the number of signals expected in the proton spin decoupled 13C spectrum of 3-hexanone, CH3CH2COCH2CH2CH3.
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the number of signals expected (disregarding splitting) in the 1H spectrum of m-xylene (1,3-dimethylbenzene).
Correct Answer

verified
Correct Answer
verified
Essay
Give one reason why 13C NMR is less sensitive than 1H NMR.
Correct Answer

verified
Natural isotopic abundance of ...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Predict the number of distinct quartets expected in the off-resonance decoupled 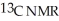 spectrum of the compound shown below.
spectrum of the compound shown below. 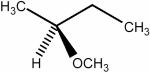
Correct Answer

verified
Correct Answer
verified
Essay
Deduce the identity of the following compound from the spectral data given.
C4H8O2: 1H NMR, δ 1.23 (3H, triplet), 2.00 (3H, singlet), 4.02 (2H, quartet) (ppm); IR, 2980, 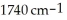
Correct Answer

verified
Correct Answer
verified
Short Answer
What multiplicities are observed in the off-resonance decoupled 13C spectrum of 2,3-dimethyl-but-2-ene?
Correct Answer

verified
a singlet ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Deduce the identity of the following compound from the 1H NMR data given. C6H8O4: δ 3.9 (6H, singlet), 6.1 (2H, singlet) (ppm)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound generates positive peaks for the carbonyl in both its DEPT-90 and DEPT-135 spectra?
A) CH3CH2CHO
B) CH3CH2COCH3
C) CH3CO2CH2CH3
D) CH3CH2CONH2
E) H2CO
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 130
Related Exams