A) positron decay.
B) alpha decay.
C) beta decay.
D) gamma decay.
E) fission.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotopes 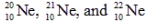 are all stable, while
are all stable, while 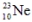 is radioactive. The mode of decay for
is radioactive. The mode of decay for 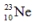 is most likely to be
is most likely to be
A) positron decay.
B) α decay.
C) ![]() decay.
decay.
D) electron capture.
E) β decay.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 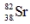 will decay through
will decay through
A) α decay.
B) β decay.
C) decay.
D) electron capture.
E) spontaneous fission.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is definitely unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with a high value of N/Z will tend to decay through
A) α decay.
B) β decay.
C) positron decay.
D) electron capture.
E) ![]() decay.
decay.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the specific activity (in Ci/g) of an isotope if 3.56 mg emits 4.26 × 108 β particles per second?
A) 0.003232 Ci/g
B) 0.0115 Ci/g
C) 0.309 Ci/g
D) 3.23 Ci/g
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following nuclei has a magic number of neutrons and/or protons?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with Z > 83, which lies close to the band of stability, will generally decay through
A) α decay.
B) β decay.
C) ![]() decay.
decay.
D) positron decay.
E) electron capture.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Palladium-107 undergoes β decay (t1/2 = 6.5 × 105 yr) to form silver-107. How long will it take for 0.150 mol of silver-107 to form from 1.25 mol of palladium-107?
A) 2.0 × 107 y
B) 1.4 × 107 y
C) 1.2 × 106 y
D) 8.3 × 105 y
E) 1.2 × 105 y
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a subatomic particle closely related to the positron?
A) proton
B) electron
C) negatron
D) neutron
E) neutrino
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the missing species in the following nuclear transmutation. 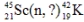
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 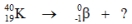
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 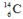 is unstable. This is predictable because
is unstable. This is predictable because
A) N/Z ≠ 1.
B) N/Z is relatively low and Z < 20.
C) N/Z is relatively large and Z < 20.
D) Z is small.
E) N is large.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following materials is put into a nuclear reactor to slow the chain reaction?
A) heavy water
B) moderators
C) control rods
D) reflectors
E) chlorine
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following isotopes is most likely to be unstable?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 55-kg person exposed to thorium-234 receives 7.5 × 104 β particles, each with an energy of 1.6 × 10-14 J. How many rads does the person receive?
A) 2.1 × 10-19
B) 1.2 × 10-17
C) 2.2 × 10-9
D) 1.2 × 10-9
E) None of these choices are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium-39 undergoes positron decay. Each positron carries 5.49 MeV of energy. How much energy will be emitted when 0.0025 mol of calcium-39 decays?
A) 13.2 kJ
B) 1.32 × 104 kJ
C) 1.32 × 106 kJ
D) 1.32 × 109 kJ
E) None of these choices are correct.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In living organisms, C-14 atoms disintegrate at a rate of 15.3 atoms per minute per gram of carbon. A charcoal sample from an archaeological site has a C-14 disintegration rate of 9.16 atoms per minute per gram of carbon. Estimate the age of this sample. The half-life of C-14 is 5730 years.
A) 3170 years
B) 3430 years
C) 4020 years
D) 4790 years
E) 6750 years
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An 85-kg person exposed to barium-141 receives 2.5 × 105 β particles, each with an energy of 5.2 × 10-13 J. How many rads does the person receive?
A) 2.4 × 10-20
B) 1.5 × 10-7
C) 1.8 × 10-16
D) 6.1 × 10-15
E) None of these choices are correct.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gamma-rays and X-rays interact with matter, causing
A) formation of ions, but no free radicals.
B) formation of free radicals, but no ions.
C) nuclear transmutation reactions.
D) formation of ions and free radicals.
E) formation of ions and nuclear transmutation reactions.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 81
Related Exams