A) 2.08 × 10- 4
B) 4.80 × 103
C) 7.10 × 107
D) 2.31 × 105
E) 4.33 × 10- 6
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elementary reaction 2NO2 (g) → 2NO (g) + O2 (g) Is second order in NO2 and the rate constant at 501 K is 7.93 × 10- 3 M- 1s- 1. The reaction half- life at this temperature when [NO2]0 = 0.450 M is s.
A) 126
B) 0.011
C) 3.6 × 10- 3
D) 280
E) 87
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
SO2Cl2 decomposes in the gas phase by the reaction SO2Cl2 (g) →SO2 (g) + Cl2 (g) The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 10- 6 s- 1at 600 K. A vessel is charged with 2.4 atm of SO2Cl2 at 600 K. The partial pressure of SO2Cl2 at 3.0 × 105 s is Atm)
A) 1.4 × 105
B) 0.76
C) 0.29
D) 2.2
E) 0.98
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A - B is first order in [A]. Consider the following data. ![The reaction A - B is first order in [A]. Consider the following data. -The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>. A) 0.46 B) 14 C) 3.0 × 10<sup>-</sup><sup> </sup><sup>2</sup><sup> </sup> D) 4.0 × 10<sup>2</sup><sup> </sup> E) 6.9 × 10<sup>-</sup><sup> </sup><sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f66_6237_ac95_4f8cb6bbc521_TB1819_00.jpg) -The rate constant for this reaction is s- 1.
-The rate constant for this reaction is s- 1.
A) 0.46
B) 14
C) 3.0 × 10- 2
D) 4.0 × 102
E) 6.9 × 10- 2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The average rate disappearance of A between 20 s and 30 s is mol/s.
A) 5.0 × 10- 4
B) 0.15
C) 1.6 × 10- 2
D) 670
E) 1.5 × 10- 3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: ![A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: -How many moles of B are present at 10 s? A) 0.220 B) 0.110 C) 1.4 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup> D) 0.014 E) 0.011](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f6a_59e3_ac95_59e6cc273c18_TB1819_00.jpg) -How many moles of B are present at 10 s?
-How many moles of B are present at 10 s?
A) 0.220
B) 0.110
C) 1.4 × 10- 3
D) 0.014
E) 0.011
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The minimum energy to initiate a chemical reaction is the .
Correct Answer

verified
Correct Answer
verified
Short Answer
The relationship of absorbed light to the concentration of the substance absorbing the light is governed by .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of a reaction depends on _.
A) collision frequency
B) collision orientation
C) collision energy
D) all of the above
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
A + B - P  -The order of the reaction in B is _ .
-The order of the reaction in B is _ .
A) 1
B) 2
C) 3
D) 4
E) 0
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: ![A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: -The average rate of appearance of B between 20 s and 30 s is mol/s. A) +5.0 × 10<sup>-</sup><sup> </sup><sup>4</sup> B) +1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) - 1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> D) - 7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup> E) +7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f6c_0796_ac95_8f4117d03f28_TB1819_00.jpg) -The average rate of appearance of B between 20 s and 30 s is mol/s.
-The average rate of appearance of B between 20 s and 30 s is mol/s.
A) +5.0 × 10- 4
B) +1.5 × 10- 3
C) - 1.5 × 10- 3
D) - 7.3 × 10- 3
E) +7.3 × 10- 3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) 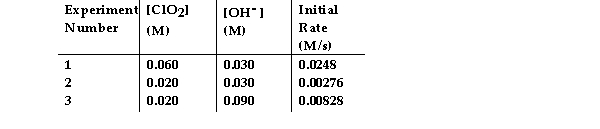 -What is the order of the reaction with respect to OH- ?
-What is the order of the reaction with respect to OH- ?
A) 0
B) 1
C) 2
D) 3
E) 4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g) At the start of the experiment, there are 0.200 mol of reactant (CH3NC) and 0 mol of product (CH3CN) in the reaction vessel. After 25 min of reaction, 0.108 mol of reactant (CH3NC) remain. The average rate of decomposition of methyl isonitrile, CH3NC, in this 25 min period is mol/min.
A) 0.54
B) 3.7 × 10- 3
C) 4.3 × 10- 3
D) 0.092
E) 2.3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: 3A - 2B The average rate of appearance of B is given by O[B]/Ot. Comparing the rate of appearance of B and the rate of disappearance of A, we get O[B]/Ot = × (- O[A]/Ot) .
A) +1
B) - 2/3
C) +3/2
D) +2/3
E) - 3/2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the rate law for the reaction ![If the rate law for the reaction is first order in A and second order in B, then the rate law is rate = . A) k[A]<sup>2</sup>[B]<sup>2</sup><sup> </sup> B) k[A][B] C) k[A][B]<sup>2</sup><sup> </sup> D) k[A]<sup>2</sup>[B]<sup>3</sup><sup> </sup> E) k[A]<sup>2</sup>[B]](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f6a_0bc2_ac95_7129822a4a55_TB1819_00.jpg) is first order in A and second order in B, then the rate law is rate = .
is first order in A and second order in B, then the rate law is rate = .
A) k[A]2[B]2
B) k[A][B]
C) k[A][B]2
D) k[A]2[B]3
E) k[A]2[B]
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2NO2 → 2NO + O2 In a particular experiment at 300°C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of disappearance of NO2 for this period is _ M/s.
A) 0.35
B) 1.8 × 10- 3
C) 3.5 × 10- 3
D) 7.0 × 10- 3
E) 3.5 × 10- 5
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) →2CO2 (g) + 2H2O (g) When the rate of disappearance of O2 is 0.28 M s- 1, the rate of appearance of CO2 is _ _ M s- 1.
A) 0.84
B) 0.42
C) 0.56
D) 0.093
E) 0.19
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The primary source of the specificity of enzymes is .
A) their polarity, which matches that of their specific substrate
B) their locations within the cell
C) their shape, which relates to the lock- and- key model
D) their bonded transition metal, which is specific to the target substrate
E) their delocalized electron cloud
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Reaction rates are affected by reactant concentrations and temperature. This is accounted for by the .
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of molecules that participate as reactants defines the of the reaction.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 110
Related Exams