A) -2
B) +2
C) +6
D) +4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the accompanying partial activity series, predict which element or ion will react with cobalt, Co. Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) Zn
B) Fe2+
C) Ni
D) Cu2+
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Knowing that sodium and potassium react violently with water, predict which element will also react with water.
A) cesium
B) aluminum
C) zinc
D) It is impossible to predict accurately from the information given.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of half-reactions takes place in a fuel cell?
A) reduction: O2 + 4 H+ + 4 e- 2H2O; oxidation: H2 2 H+ + 2 e-
B) reduction: H2 2 H+ + 2 e-; oxidation: O2 + 4 H+ + 4 e- 2 H2O
C) reduction: O2 + 4 H+ + 4 e- 2H2O; oxidation: 2 H+ + 2 e- H2
D) reduction: H2 2 H+ + 2 e-; oxidation: 2 H2O O2 + 4H+ + 4 e-
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the context of chemical reactions, oxidation refers to the:
A) gain of electrons.
B) loss of electrons.
C) loss of oxygen.
D) loss of water.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to identify the components labeled 1, 2, 3, and 4 respectively. 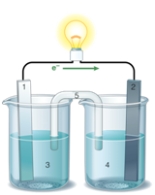 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron, nickel, iron ion solution, nickel ion solution
B) nickel, iron, iron ion solution, nickel ion solution
C) nickel, iron, nickel ion solution, iron ion solution
D) iron, nickel, nickel ion solution, iron ion solution
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation half-reaction for the reaction of zinc with hydrochloric acid is:
A) Zn2+ + 2 e- Zn.
B) Zn Zn2+ + 2 e-.
C) H2 2 H+ + 2 e-.
D) 2 H+ + 2 e- H2.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the balanced net ionic equation for the reaction consisting of the two half-reactions given. Cu Cu2+ + 2e- Au3+ + 3e- Au
A) Cu (s) + Au3+ (aq) Cu2+ (aq) + Au (s)
B) 3 Cu (s) + 2 Au3+ (aq) 3 Cu2+ (aq) + 2 Au (s)
C) 2 Au (s) + 3 Cu2+ (aq) 2 Au3+ (aq) + 3 Cu (s)
D) Au (s) + Cu2+ (aq) Au3+ (aq) + Cu (s)
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the accompanying drawing of an electrochemical cell, identify the components labeled 1, 2, and 5, respectively. 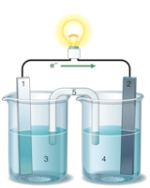
A) salt bridge, anode, cathode
B) salt bridge, cathode, anode
C) cathode, anode, salt bridge
D) anode, cathode, salt bridge
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the partial activity series included to determine which reaction will NOT occur spontaneously. Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) Fe (s) + Co2+ (aq) Fe2+ (aq) + Co (s)
B) Zn (s) + Ni2+ (aq) Zn2+ (aq) + Ni (s)
C) Cu (s) + Fe2+ (aq) Cu2+ (aq) + Fe (s)
D) Ni (s) + Cu2+ (aq) Ni2+ (aq) + Cu (s)
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of sulfur in sulfur trioxide, SO3?
A) -2
B) +2
C) +6
D) +4
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which pair of half-reactions could be used to electroplate silver onto iron?
A) reduction: Ag+ + e- Ag; oxidation: Ag Ag+ + e-
B) reduction: Ag Ag+ + e-; oxidation: Ag+ + e- Ag
C) reduction: Fe2+ + 2 e- Fe; oxidation: Fe Fe2+ + 2 e-
D) reduction: Fe Fe2+ + 2 e-; oxidation: Fe2+ + 2 e- Fe
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider an electrochemical cell consisting of zinc and cobalt half-cells. Use the partial activity series included to determine the direction in which the electrons in the wire will flow. Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) from the zinc anode to the cobalt cathode
B) from the zinc cathode to the cobalt anode
C) from the cobalt anode to the zinc cathode
D) from the cobalt cathode to the zinc anode
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the accompanying partial activity series, which element(s) can reduce copper(II) to elemental copper? Zn Zn2+ + 2e- Fe Fe2+ + 2e- Co Co2+ + 2e- Ni Ni2+ + 2e- Cu Cu2+ + 2e-
A) nickel
B) iron
C) zinc
D) nickel, iron, and zinc
E) None of these elements can reduce copper(II) to elemental copper.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the accompanying partial activity series, which element(s) will NOT react with acid? Zn Zn2+ + 2e- Fe Fe2+ + 2e- Ni Ni2+ + 2e- H2 2H+ + 2e- Cu Cu2+ + 2e- Ag Ag+ + e-
A) nickel
B) iron
C) silver
D) nickel, iron, and silver
E) All of these elements will react with acid.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which example does NOT illustrate a redox reaction?
A) metal and nonmetal
B) double displacement
C) single displacement
D) combustion
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the coefficients of the balanced equation that represents the reaction in a magnesium/iron electrochemical cell? Mg (s) + Fe2+ (aq) Fe (s) + Mg2+ (aq)
A) 1, 1, 1, 1
B) 1, 2, 2, 1
C) 2, 1, 1, 2
D) 1, 2, 1, 2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of sulfur in the sulfite ion, SO32-?
A) -2
B) +2
C) +6
D) +4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to select the half-reaction represented by the LEFT side of the cell. 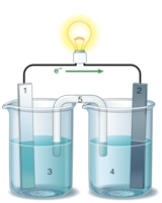 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) Fe Fe2+ + 2e-
B) Ni Ni2+ + 2e-
C) Fe2+ + 2e- Fe
D) Ni2+ + 2e- Ni
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the coefficients of the balanced equation that represents the reaction in a magnesium/aluminum electrochemical cell? Mg (s) + Al3+ (aq) Al (s) + Mg2+ (aq)
A) 1, 1, 1, 1
B) 3, 2, 2, 3
C) 2, 3, 3, 2
D) 1, 2, 1, 3
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 52
Related Exams