B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct name for the compound Mn2O3?
A) dimanganese trioxide
B) manganate ion
C) manganese(III) oxide
D) manganese(II) oxide
E) manganese oxide
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for a compound composed of Ca2+ ions and PO43- ions?
A) CaPO4
B) Ca2(PO4) 3
C) Ca3(PO4) 2
D) Ca(PO4) 2
E) Ca(PO4) 3
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the compound with the name manganese(II) phosphate?
A) Mn3(PO4) 2
B) Mn2(PO4) 3
C) MnPO4
D) Mn3P2
E) Mn2P3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for the chlorate ion is ClO3-.What is the formula for the perchlorate ion?
A) ClO4-
B) ClO2-
C) ClO-
D) ClO32-
E) PClO3-
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
From their position in the periodic table, we would predict that both oxygen and sulfur would have a charge of 2- in a binary ionic compound.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aluminum sulfate, calcium oxide, and water react together in a process used to remove solids from treated water.Which set of formulas is correct?
A) AlSO4, Ca2O, H2O
B) Al3(SO4) 2, Ca2O, H2O
C) Al2(SO4) 3, Ca2O, H2O2
D) Al2(SO4) 3, CaO2, H2O2
E) Al2(SO4) 3, CaO, H2O
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the compound with the name aluminum sulfide?
A) AlS
B) Al3S2
C) Al2S3
D) AlSO4
E) Al2(SO4) 3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The images in the figure represent aqueous solutions of ionic compounds.Which could correspond to Na2S? 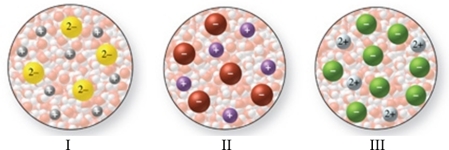
A) I only
B) II only
C) III only
D) I and III
E) II and III
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formulas for a compound containing the Cr2+ ion is incorrect?
A) CrClO4
B) CrSO4
C) CrO
D) CrS
E) CrCl2
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for calcium nitrate is:
A) CaNO3
B) Ca2NO3
C) Ca(NO3) 2
D) Ca(NO3) 3
E) Ca(NO2) 2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of formulas is correct for the compounds ammonia, nitric acid, and nitrous acid, respectively?
A) NH3, HN, HNO
B) NH4, HNO2, HNO3
C) NH3, HNO2, HNO3
D) NH3, HNO3, HNO2
E) NH4, HNO3, HNO2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 112 of 112
Related Exams