A) Δ H° > 0, Δ S° > 0
B) Δ H° > 0, Δ S° < 0
C) Δ H° < 0, Δ S° > 0
D) Δ H° < 0, Δ S° < 0
E) None of these choices are correct.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrogen sulfide decomposes according to the following reaction 2H2S(g) → 2H2(g) + S2(g) For this reaction at 298K ΔS° = 78.1 J/K, ΔH° = 169.4 kJ, and ΔG° = 146.1 kJ. What is the value of ΔG° at 900 K?
A) −69881 kJ
B) 48.4 kJ
C) 99.1 kJ
D) 240 kJ
E) 441 kJ
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elemental boron can be formed by reaction of boron trichloride with hydrogen. BCl3(g) + 1.5H2(g) → B(s) + 3HCl(g)
Calculate ΔG° for the reaction.
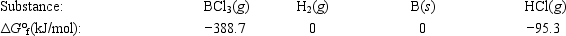
A) −293.4 kJ
B) 293.4 kJ
C) −102.8 kJ
D) 102.8 kJ
E) None of these choices are correct.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine. The data refer to 298 K. SO2(g) + Cl2(g) → SO2Cl2(g)
 What is the value of ΔG° for this reaction at 600 K?
What is the value of ΔG° for this reaction at 600 K?
A) −162.8 kJ
B) −40.1 kJ
C) −28.4 kJ
D) 28.4 kJ
E) 162.8 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a chemical reaction to be spontaneous at all temperatures, which of the following conditions must be met?
A) Δ S° > 0, Δ H° > 0
B) Δ S° > 0, Δ H° < 0
C) Δ S° < 0, Δ H° < 0
D) Δ S° < 0, Δ H° > 0
E) It is not possible for a reaction to be spontaneous at all temperatures.
G) B) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to 25°C. CH4(g) + 2H2O(g) ⇄ CO2(g) + 4H2(g)

A) 8.2 × 10 19
B) 0.96
C) 0.58
D) 1.2 × 10 −20
E) 1.4 × 10 −46
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given: H2O(l) → H2O(s) ΔH° = −6.02 kJ at 273K Calculate the entropy change of the surroundings (ΔSsurr) when one mole of water freezes at 0°C and a pressure of one atmosphere.
A) 22.1 J/K
B) −22.1 J/K
C) 397 J/K
D) −397 J/K
E) 0.022 J/K
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
As a chemical reaction proceeds toward equilibrium, the free energy of the system decreases.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following quantities used in thermodynamics: E, H, q, w, S, G. How many of them are state functions?
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"A diamond is forever" is one of the most successful advertising slogans of all time. But is it true? For the reaction shown below, calculate the standard free energy change at 298K and determine whether or not a diamond is "forever". C(diamond) → C(graphite) Data: ΔHf°(diamond) = 1.895 kJ/mol; S°(diamond) = 2.337 J mol−1K−1; S°(graphite) = 5.740 J mol−1K−1.
A) Δ G° = 2.19 kJ; forever
B) Δ G° = −1.90 kJ; not forever
C) Δ G° = −2.90 kJ; not forever
D) Δ G° = 1.90 kJ; forever
E) Δ G° = < −1000 kJ; not forever
G) All of the above
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A sample of water is heated at a constant pressure of one atmosphere. Initially, the sample is ice at 260 K, and at the end the sample consists of steam at 400 K. In which of the following 5K temperature intervals would there be the greatest increase in the entropy of the sample?
A) From 260 K to 265 K
B) From 275 K to 280 K
C) From 360 K to 365 K
D) 370 K to 375 K
E) From 395 K to 400 K
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true for pure oxygen gas, O2(g) at 25°C?
A) Δ H° f > 0
B) Δ H° f < 0
C) Δ G° f > 0
D) Δ G° f < 0
E) S° > 0
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of these pairs will the entropy of the first substance be greater than that of the second? Assume P and T are the same for each pair, unless stated otherwise.
A) 1 mole of F 2( g) ; 1 mole of Cl 2( g)
B) 1 mole of I 2( s) ; 1 mole of I 2( g)
C) 1 mole of CaCO 3( s) ; 1 mole of CaO( s) plus 1 mole of CO 2( g)
D) 1 mole of H 2( g) at 25°C; 1 mole of H 2( g) at 50°C
E) 1 mole of O 3( g) ; 1 mole of O 2( g)
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following values is based on the Third Law of Thermodynamics?
A) Δ H° f = 0 for Al( s) at 298 K
B) Δ G° f = 0 for H 2( g) at 298 K
C) S° = 51.446 J/(mol·K) for Na( s) at 298 K
D) q sys < 0 for H 2O( l) → H 2O(s) at 0°C
E) None of these choices are correct.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the free energy change, ΔG°, for the equilibrium between hydrogen iodide, hydrogen, and iodine at 453°C? Kc = 0.020 2HI(g) ⇄ H2(g) + I2(g)
A) 6.4 kJ
B) 8.8 kJ
C) 15 kJ
D) 19 kJ
E) 24 kJ
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You are given pure samples of ammonia, NH3(g) , and nitrogen trifluoride, NF3(g) . What prediction would you make concerning their standard molar entropies at 298 K?
A) S° ammonia > S° nitrogen trifluoride
B) S° ammonia < S° nitrogen trifluoride
C) S° ammonia ≈ S° nitrogen trifluoride
D) Other conditions need to be specified before a reliable prediction can be made.
E) Even if more conditions are specified, a reliable prediction cannot be made.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following phase changes decreases the entropy of the system?
A) Melting
B) Heating a gas
C) Vaporization
D) Condensation
E) Sublimation
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The term microstate refers to the energy state of a single molecule in a system of many molecules.
B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
A reaction has ΔG = 10.0 kJ and ΔG° = 15.0 kJ at a temperature of 50°C. Calculate the value of the reaction quotient 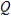 under these conditions.
under these conditions.
A) 0.16
B) 9.1 × 10 −5
C) 1.1 × 10 4
D) 6.4
E) 6.0 × 10 −6
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following should have the greatest molar entropy at 298 K?
A) CH 4( g)
B) H 2O( l)
C) NaCl( s)
D) N 2O 4( g)
E) H 2( g)
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 85
Related Exams