B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination of formula and name is incorrect?
A) Na+ = sodium ion
B) Ca2+ = calcium ion
C) N2- = nitride ion
D) F- = fluoride ion
E) O2- = oxide ion
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following combinations of elements in a compound would be molecular?
A) sodium and nitrogen
B) calcium and oxygen
C) nitrogen and oxygen
D) potassium and chlorine
E) sodium and sulfur
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination of formula and name is incorrect?
A) K+ = potassium ion
B) I- = iodide ion
C) Mg+ = magnesium ion
D) S2- = sulfide ion
E) N3- = nitride ion
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
A molecular compound is composed of atoms of a metal and a nonmetal.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Methane, CH4, and acetic acid, CH3CO2H, are both acids because they both contain hydrogen. 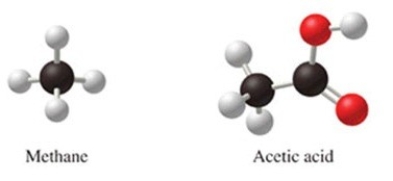
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compound names is not correct?
A) strontium dinitrate
B) sodium oxide
C) copper(II) carbonate
D) potassium permanganate
E) silver chloride
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
From their position in the periodic table, we would predict that both oxygen and sulfur would have a charge of 2− in a binary ionic compound.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name pairs is incorrect?
A) K2SO4 potassium sulfate
B) Na3N sodium nitride
C) Li2CO3 lithium carbonate
D) Fe2O3 iron(II) oxide
E) Cu2S copper(I) sulfide
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for the permanganate ion is:
A) MnO4-
B) MnO22-
C) MnO3-
D) MnO32-
E) MnO42-
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for a compound composed of Ca2+ ions and PO43− ions?
A) CaPO4
B) Ca2(PO4) 3
C) Ca3(PO4) 2
D) Ca(PO4) 2
E) Ca(PO4) 3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formula/name pairs is incorrect?
A) MnO manganese(II) oxide
B) CaO calcium oxide
C) MgCl2 magnesium chloride
D) AlP aluminum phosphide
E) Li2SO3 dilithium sulfite
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure shows all but which of the following? 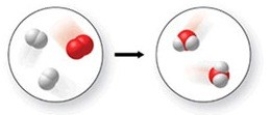
A) two elements and a compound
B) a chemical reaction
C) one mixture and one pure substance
D) three compounds
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on common charges, which formula for an ionic compound is incorrect?
A) LiCO3
B) NaBr
C) CaO
D) SrS
E) MgCl2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound ClO3 is a dark brown solid powder that is not stable above 25ºC. Name this molecular compound.
A) chlorine trioxide
B) chlorine oxide
C) chlorate
D) chlorite
E) chlorine(VI) oxide
G) A) and D)
Correct Answer

verified
Correct Answer
verified
True/False
When naming binary molecular compounds, it is always unnecessary to use Greek prefixes.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes HCl when dissolved in water?
A) strong electrolyte
B) weak electrolyte
C) nonelectrolyte
D) exists as atoms and molecules
E) exists as molecules only
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct formula for the nitride ion?
A) N2
B) NO2-
C) NO3-
D) N23-
E) N3-
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The images in the figure represent aqueous solutions of ionic compounds. Which could correspond to BaCl2? 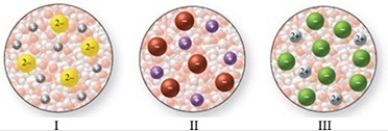
A) I only
B) II only
C) III only
D) I and III
E) II and III
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is formed when Ca(NO3) 2 dissolves in water?
A) Ca2+ + (NO3) 22-
B) Ca + (NO3) 2
C) Ca+ + 2NO3-
D) Ca2+ + 2NO3−
E) Ca2+ + 2N3- + 6O2-
G) None of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 113
Related Exams