A) one; three
B) one; five
C) three; five
D) eight; eight
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Van der Waals interactions may result when ________.
A) electrons are not symmetrically distributed in a molecule
B) molecules held by ionic bonds react with water
C) two polar covalent bonds react
D) a hydrogen atom loses an electron
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A(n) ________ has charge but negligible mass, whereas a(n) ________ has mass but no charge.
A) proton; neutron
B) neutron; proton
C) neutron; electron
D) electron; neutron
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactivity of an atom arises from
A) the average distance of the outermost electron shell from the nucleus.
B) the existence of unpaired electrons in the valence shell.
C) the sum of the potential energies of all the electron shells.
D) the potential energy of the valence shell.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A covalent chemical bond is one in which ________.
A) electrons are removed from one atom and transferred to another atom so that the two atoms become oppositely charged
B) protons and neutrons are shared by two atoms so as to satisfy the requirements of both atoms
C) outer-shell electrons of two atoms are shared so as to satisfactorily fill their respective orbitals
D) outer-shell electrons of one atom are transferred to fill the inner electron shell of another atom
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elements ⁷²Zn, ⁷⁵As, and ⁷⁴Ge have the ________.
A) same number of protons
B) same number of protons and electrons
C) same number of neutrons
D) same number of neutrons and electrons
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of nitrogen is 7. Nitrogen-15 has a greater mass number than nitrogen-14 because the atomic nucleus of nitrogen-15 contains ________.
A) 7 neutrons
B) 8 neutrons
C) 8 protons
D) 15 protons
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Can the atomic mass of an element vary?
A) No, it is fixed; otherwise a new element will be formed.
B) Yes. Adding or losing electrons will substantially change the atomic mass.
C) Yes. Adding or losing protons will change the atomic mass without forming a different element.
D) Yes. Adding or losing neutrons will change the atomic mass without forming a different element.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) Carbon, hydrogen, oxygen, and calcium are the most abundant elements of living matter.
B) Some naturally occurring elements are toxic to organisms.
C) All life requires the same essential elements.
D) A patient suffering from a goiter should not consume seafood.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure (first three rows of the periodic table) to answer the questions below.
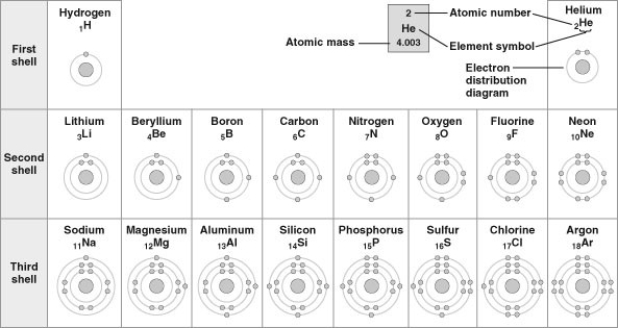 -What element does not prefer to react with other elements?
-What element does not prefer to react with other elements?
A) hydrogen
B) helium
C) beryllium
D) both hydrogen and beryllium
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a chemical reaction, the element ¹³Al will most preferably ________.
A) lose three electrons and become positively charged
B) gain five electrons and become negatively charged
C) lose five electrons and become positively charged
D) gain three electrons and become positively charged
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the term trace element, the adjective trace means that
A) the element is required in very small amounts.
B) the element can be used as a label to trace atoms through an organism's metabolism.
C) the element is very rare on Earth.
D) the element enhances health but is not essential for the organism's long-term survival.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
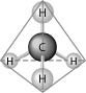 -When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
-When the atoms involved in a covalent bond have the same electronegativity, what type of bond results?
A) an ionic bond
B) a hydrogen bond
C) a nonpolar covalent bond
D) a polar covalent bond
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below. 3H₂ + N₂ 2NH₃ -Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no net effect on the concentrations of the reactants and products.
B) Concentrations of products are higher than the concentrations of the reactants.
C) There are equal concentrations of products and reactants while forward and reverse reactions continue.
D) There are equal concentrations of reactants and products, and the reactions have stopped.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen (N) is more electronegative than hydrogen (H) . Which of the following is a correct statement about the atoms in ammonia (NH₃) ?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) Ammonia has an overall positive charge.
C) Ammonia has an overall negative charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of chlorine is 17. The atomic number of magnesium is 12. What is the formula for magnesium chloride?
A) MgCl
B) MgCl₂
C) Mg₂Cl
D) MgCl₃
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms have no electric charge because they have ________.
A) uncharged neutrons in their nuclei
B) an equal number of protons and neutrons
C) an equal number of protons and electrons
D) an equal number of charged and uncharged subatomic particles
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? C₆H₁₂O₆ → ________ C₂H₆O + ________ CO₂
A) 2; 1
B) 3; 1
C) 1; 3
D) 2; 2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the questions below.
 -Based on electron configuration, which of the elements in the figure would exhibit a chemical behavior most like that of oxygen?
-Based on electron configuration, which of the elements in the figure would exhibit a chemical behavior most like that of oxygen?
A) carbon
B) nitrogen
C) sulfur
D) phosphorus
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 61
Related Exams