A) are the subatomic particles most involved in bonding behavior of atoms.
B) have a positive charge of one.
C) comprise the majority of the mass of an atom.
D) do not participate in the bonding of atoms.
E) are located in the nucleus of an atom.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Solution A increases its acidity. This means that the
A) pH of the solution has increased.
B) number of hydrogen ions has increased.
C) solution is closer to neutrality.
D) solution will now accept more protons.
E) number of hydrogen ions has decreased.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
X-rays can be used to view bones because
A) x-rays can not pass through bone.
B) x-rays pass through bone.
C) x-rays react with bone.
D) bones are less dense than soft tissue.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the ratio of products and reactants are stable, the system is in _______.
A) activation
B) steady state
C) equilibrium
E) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the material that would NOT be considered an important inorganic substances in our bodies.
A) carbon
B) iron
C) calcium
D) oxygen
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What molecule is produced as a waste product of the metabolism of glucose by cells?
A) water
B) oxygen
C) carbon monoxide
D) nitrogen
E) carbon dioxide
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following chemical reactions best represents the decomposition of ATP?
A) ATP + ADP ATP
B) ATP + energy ADP + H2O
C) ATP + H2O ADP + Pi + energy
D) ADP + ADP + ADP ATP
E) ADP + Pi + energy ATP + H2O
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an organic compound?
A) hydrochloric acid (HCl)
B) salt (NaCl)
C) sucrose (C12H22O11)
D) water (H2O)
E) None of these choices are correct.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following organic groups does DNA belong to?
A) protein
B) lipid
C) nucleic acid
D) carbohydrate
E) vitamin
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The three forms of matter are:
A) air, water, and solids.
B) solids, liquids, and gases.
C) blood, bone, and air.
D) vapor, water, and solid.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A buffer will
A) enhance changes in the pH of the solutions.
B) make a solution more acidic.
C) make a solution more basic.
D) have no effect on the pH of the solutions.
E) resist drastic changes in the pH of the solutions.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In ionic bonding,
A) electrons are transferred from one atom to another.
B) the charge of the ion does not play a role in the bond.
C) only non-polar molecules are involved.
D) two hydrogen atoms share one pair of electrons.
E) a "sea of electrons" forms.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct complementary strand to CATGTC?
A) GUACAG
B) TCGTAT
C) CATGTC
D) GTACAG
E) AGCACA
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reversible reaction, CO2 + H2O H2CO3 H+ + HCO3- , a decrease in respiration rate will increase the concentration of CO2 in the blood. What will this do to the amount of H+ in the blood?
A) H+ will decrease.
B) H+ will increase.
C) H+ will be unchanged.
E) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
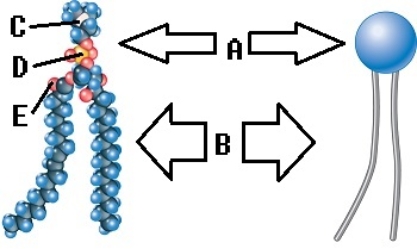 -Phospholipids are important components of the plasma membrane. What does "E" represent on the diagram?
-Phospholipids are important components of the plasma membrane. What does "E" represent on the diagram?
A) phosphorus
B) oxygen
C) nitrogen
D) polar (hydrophilic) region
E) nonpolar (hydrophobic) region
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A cation is
A) a molecule that conducts electricity when placed in solution.
B) an alteration in the three-dimensional structure of a protein.
C) a positively charged ion.
D) a combination of atoms held together by chemical bonds.
E) a negatively charged ion.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
DNA
A) contains the sugar deoxyribose.
B) assembles amino acids to make proteins..
C) is one of several amino acids.
D) must travel to ribosomes to function.
E) is a single-stranded molecule.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A substance that will increase the rate of a chemical reaction without being permanently changed is called a/an
A) oxidator.
B) reducing agent.
C) catalyst.
D) solute.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following organic groups does hemoglobin belong to?
A) nucleic acid
B) vitamin
C) lipid
D) protein
E) carbohydrate
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nitrogen bases is found in RNA but not DNA?
A) cytosine
B) adenine
C) thymine
D) uracil
E) guanine
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 168
Related Exams