A) temperature of the solvent
B) double bonds between the carbon atom and other atoms
C) polarity of the covalent bonds
D) solvent in which the organic molecule is dissolved
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following unique feature of carbon allows it to support life on Earth?
A) Carbon is hydrophobic in nature.
B) Carbon is the most abundant element on earth.
C) Carbon can form a variety of bonds in nature.
D) Carbon is the most electronegative element in the periodic table.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compared to a hydrocarbon chain where all the carbon atoms are linked by single bonds, which of the following statements best describe a hydrocarbon chain with the same number of carbon atoms but with one or more double bonds?
A) It will be more flexible in structure.
B) It will be more constrained in structure.
C) It will be more polar.
D) It will contain more hydrogen atoms.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
ATP is necessary for life because ________.
A) it tastes good
B) it is soluble in water
C) it speeds up the biological processes
D) it is the principle energy carrying molecule in a cell
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following illustrations is not a structural isomer of an organic compound with the molecular formula C6H14? For clarity, only the carbon skeletons are shown; hydrogen atoms that would be attached to the carbons have been omitted.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional group is present in this molecule? 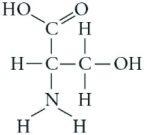
A) sulfhydryl
B) carboxyl
C) methyl
D) phosphate
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Visualize the structural formula of each of the following hydrocarbons. Which hydrocarbon has a double bond in its carbon skeleton?
A) C3H8
B) C2H6
C) C2H4
D) C2H2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which two functional groups are always found in amino acids?
A) carbonyl and amino groups
B) carboxyl and amino groups
C) amino and sulfhydryl groups
D) hydroxyl and carboxyl groups
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Miller's classic experiment demonstrated that a discharge of sparks through a mixture of gases could result in the formation of a large variety of organic compounds. Miller did not use which one of the following gases in his experiment?
A) methane
B) oxygen
C) water
D) ammonia
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the question. 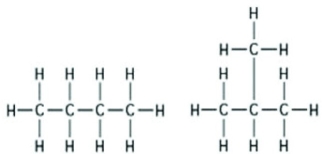 The two molecules shown in the figures are best described as ________.
The two molecules shown in the figures are best described as ________.
A) enantiomers
B) structural isomers
C) cis-trans isomers
D) chain length isomers
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the term that correctly describes the relationship between these two sugar molecules. 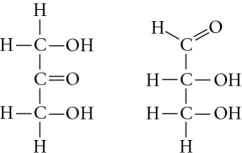
A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the following question. 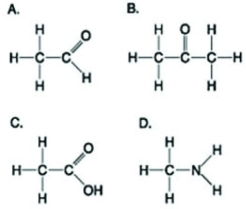 Which molecule shown has a carbonyl functional group in the form of a ketone?
Which molecule shown has a carbonyl functional group in the form of a ketone?
A) A
B) B
C) C
D) D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A carbon atom is most likely to form what kind of bond(s) with other atoms?
A) ionic
B) hydrogen
C) covalent
D) carbon is an inert element
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following identifies the chemical relationship between glucose and fructose?
A) They are isotopes.
B) They are enantiomers.
C) They are cis-trans isomers.
D) They are structural isomers.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following functional groups gives amino acids their acidic character?
A) amino
B) carbonyl
C) carboxyl
D) phosphate
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the following question. 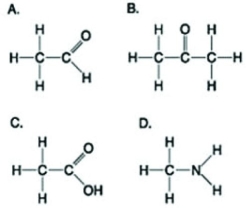 Which molecule(s) shown is (are) ionized in a cell?
Which molecule(s) shown is (are) ionized in a cell?
A) A
B) B and D
C) C and D
D) D
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the question. 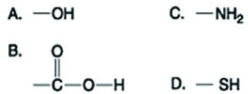 Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?
Which functional group shown in the figure can accept protons and raise the pH of the surrounding solution?
A) A
B) B
C) C
D) D
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following explains why the valency of carbon is 4 even though it has 6 electrons?
A) donates its 2 electrons to another atom
B) shares its 2 electrons and bonds with another atom
C) has 4 electrons in its first shell and 2 in the second shell
D) has 2 electrons in its first shell and 4 in the second shell
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the figures to answer the following question. 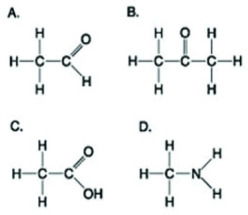 Which molecule shown has at least one carbon atom attached to three different chemical groups?
Which molecule shown has at least one carbon atom attached to three different chemical groups?
A) A
B) B
C) D
D) A and B
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about ADP/ATP is true?
A) ADP contains more energy than ATP.
B) Following hydrolysis, ATP can release one phosphate, whereas ADP cannot.
C) ADP can have two positive charges.
D) ATP can have four negative charges.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 52
Related Exams