A) 69.5 atm
B) 5.63 atm
C) 40.0 atm
D) 0.625 atm
E) 4.44 atm
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of neon occupy a volume of 14.3 L at STP?
A) 32.0 moles
B) 6.45 moles
C) 1.57 moles
D) 36.7 moles
E) 0.638 moles
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of argon gas at 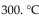 and 50.0 atm pressure is cooled in the same container to a temperature of
and 50.0 atm pressure is cooled in the same container to a temperature of 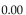
 What is the new pressure, if volume and amount of gas do not change?
What is the new pressure, if volume and amount of gas do not change?
A) 105 atm
B) 42.7 atm
C) 45.5 atm
D) 54.9 atm
E) 23.8 atm
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A tank contains helium gas at 490 mmHg , nitrogen gas at 0.75 atm, and neon at 520 torr. What is the total pressure in atm?
A) 0.55 atm
B) ![]()
C) 1.5 atm
D) 2.1 atm
E) 1600 atm
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the kinetic molecular theory of gas behavior, particles of a gas tend to move ________ in ________.
A) rapidly; straight paths
B) slowly; curve paths
C) slowly; straight paths
D) rapidly; curved paths
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At STP, temperature and pressure have the values of
A) 0 K and 1 atm.
B) 760 K and 273 atm.
C) 0 K and 760 mmHg.
D) 273 K and 1 mmHg.
E) 273 K and 760 mmHg.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to Avogadro's law,
A) the volume of a gas depends only on the number of moles in the sample.
B) the volume of a gas is directly related to the number of moles at constant temperature and pressure.
C) the volume of a gas is inversely related to the number of moles at standard temperature and pressure.
D) the volume of a gas is inversely related to the number of moles at constant temperature and pressure.
E) the volume of a gas depends only on the temperature and pressure.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The unit of 1 atmosphere used to describe the pressure of a gas is equal to
A) 200 mmHg.
B) 1 mmHg .
C) 100 mmHg.
D) 760 mmHg.
E) 600 mmHg.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas sample contains 4.0 g of CH4 and 2.0 g of He. What is the volume of the sample at STP?
A) 130 L
B) 17 L
C) 5.6 L
D) 30. L
E) 11 L
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The volume of a gas with a pressure of 1.2 atm increases from 1.0 L to 4.0 L. What is the final pressure of the gas, assuming no change in amount of gas or temperature?
A) 0.30 atm
B) 4.8 atm
C) 1.0 atm
D) 1.2 atm
E) 3.3 atm
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atmospheric pressure on a rainy day is usually ________ air pressure on a sunny day.
A) higher than
B) lower than
C) the same as
E) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The total pressure in a mixture of gases is equal to the partial pressure(s) of
A) all the gases added together.
B) the gas with the highest molecular weight.
C) the gas with the smallest number of moles.
D) the gas with the greatest number of moles.
E) the gas that occupies the largest volume.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Gay-Lussac's law is usually written as ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of nitrogen gas had a volume of 500 .mL, a pressure in its closed container of 740 torr, and a temperature of 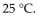 What was the new volume of the gas when the temperature was changed to
What was the new volume of the gas when the temperature was changed to 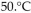 and the new pressure was 760 torr, if the amount of gas does not change?
and the new pressure was 760 torr, if the amount of gas does not change?
A) 400 mL
B) 240 mL
C) 450 mL
D) 970 mL
E) 530 mL
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As you rise higher in Earth's atmosphere, the atmospheric pressure
A) decreases.
B) increases.
C) remains the same.
E) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 5.00-L tank contains helium gas at 1.50 atm. What is the pressure of the gas in mmHg?
A) 760. mmHg
B) 7.50 mmHg
C) 1.50 mmHg
D) 507 mmHg
E) 1140 mmHg
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
A barometer is usually filled with ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which measurement describes the pressure of a gas?
A) 725 mmHg
B) 1.2 g/L
C) 2.5 L
D) 0.45 moles
E) 315 K
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the kinetic theory of gases, particles of a gas
A) move slowly.
B) are very large.
C) decrease kinetic energy as temperature increases.
D) are very far apart.
E) lose their valence electrons.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant temperature, a sample of helium at 760. torr in a closed container was compressed from 5.00 L to 3.00 L, with no change in amount of gas or temperature. What was the new pressure exerted by the helium on Its container?
A) 15.0 torr
B) 2280 torr
C) 800. torr
D) 3820 torr
E) 1270 torr
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 84
Related Exams