A) They are shiny.
B) They react vigorously with water.
C) They are good conductors of heat.
D) They are good conductors of electricity.
E) Most of them are liquids at room temperature.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a neutral atom with 30 protons and 34 neutrons. The mass number for this atom is
A) 30.
B) 32.
C) 34.
D) 64.
E) 94.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes are atoms of the same element that have
A) the same atomic numbers but different numbers of electrons.
B) the same atomic number but different numbers of neutrons.
C) the same atomic mass but different numbers of protons.
D) the same atomic numbers but different numbers of protons.
E) different atomic numbers.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a neutral atom with 30 protons and 34 neutrons. The number of electrons in this atom is
A) 30.
B) 32.
C) 34.
D) 64.
E) 94.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A) carbon, phosphorus, oxygen, helium
B) carbon, potassium, oxygen, helium
C) calcium, phosphorus, oxygen, hydrogen
D) carbon, potassium, oxygen, hydrogen
E) calcium, phosphorus, oxygen, helium
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the symbol of the element in Period 4 and Group 2 A (2) ?
A) Mg
B) Be
C) Si
D) C
E) Ca
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Valence electrons are electrons located
A) throughout the atom.
B) in the first three energy levels of an atom.
C) in the innermost energy level of an atom.
D) in the nucleus of an atom.
E) in the outermost energy level of an atom.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the abbreviated electron configuration for nickel (atomic number 28) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electron configurations is impossible?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct symbol for the element. -iron
A) Ir
B) In
C) Fs
D) Fe
E) FE
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ionization energy is
A) the energy an ion acquires from an electron.
B) higher for potassium than for lithium.
C) highest for metals in Group 1A (1) .
D) the energy needed to remove an electron from the outermost energy level.
E) higher for chlorine than for fluorine.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives the correct numbers of protons, neutrons, and electrons, respectively, in a neutral atom of 11850 Sn?
A) 68 protons, 68 neutrons, 50 electrons
B) 118 protons, 50 neutrons, 118 electrons
C) 118 protons, 118 neutrons, 50 electrons
D) 50 protons, 50 neutrons, 50 electrons
E) 50 protons, 68 neutrons, 50 electrons
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The primary substances of which all other things are composed are
A) protons.
B) compounds.
C) electrons.
D) molecules.
E) elements.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of electron levels in a magnesium atom is
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a noble gas?
A) chlorine
B) argon
C) oxygen
D) nitrogen
E) bromine
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the symbol of the element in Group 4A (14) and Period 2?
A) Ca
B) C
C) Si
D) Be
E) Mg
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ca is the symbol for
A) cadmium.
B) copper.
C) cobalt.
D) calcium.
E) carbon.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the element with the abbreviated electron configuration 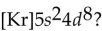
A) Pd
B) Kr
C) Pt
D) Xe
E) Ni
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of neutrons in an atom is equal to
A) the mass number + the atomic number.
B) the atomic number.
C) the number of protons.
D) the mass number.
E) the mass number - the atomic number.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the elements: B, C, F, Li, and Na, the element with the largest atomic radius is
A) B.
B) C.
C) F.
D) Li.
E) Na.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 83
Related Exams