Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium chlorate, an ingredient in many common herbicides, has sodium, chlorine and oxygen atoms in the ratio 1:1:3, respectively. What is the formula unit for sodium chlorate?
A) NaCO3
B) SoClO3
C) NaClO3
D) none of these
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Naturally occurring lithium (Li) consists of only two isotopes, Li-6 (6.02 amu) and Li-7 (7.02 amu) , where the exact isotopic masses are given in parentheses. Use the periodic table and determine which isotope is present in the larger percentage in the natural element.
A) ![]()
B) ![]()
C) The percentage of each isotope is about the same.
D) The relative percent abundance cannot be determined from the information available.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the chemical symbol for the elements that is in period 2 and group 8A of the periodic table.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a particular solid sample is examined under a microscope, it is observed that there are regions that are black and regions which are yellow. What type of matter is this sample?
A) a compound
B) an element
C) a homogeneous mixture
D) a heterogeneous mixture
F) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
An elements with an electron arrangement of: shell 1: 2 electrons shell 2: 8 electrons shell 3: 18 electrons shell 4: 18 electrons shell 5: 7 electrons would have 1 valence electron.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate the number of dots that should be placed around the Lewis symbol for the following element. Use an integer: 1, 2, 3, etc. as appropriate. Br
Correct Answer

verified
Correct Answer
verified
Short Answer
Enter the chemical symbol of the element in the blank. An element in period 3 has the Lewis structure: 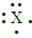 X should be replaced with what chemical symbol?
Chemical symbol:_____________________.
X should be replaced with what chemical symbol?
Chemical symbol:_____________________.
Correct Answer

verified
Correct Answer
verified
True/False
The atomic weight of phosphorus (P) is 30.91 amu or about 31 amu. This indicates that each P atom consists of 15 protons and 16 neutrons.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Electron shells define a region in space around the nucleus occupied by certain electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. There are _______mol is 54.05 g of boron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A person drinks 1900 g of water, H2O, per day. How many moles of water did they consume?
A) ![]() mol
mol
B) 0.009 mol
C) 105 mol
D) 18.02 mol
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the periodic table to determine about how many helium atoms (He) on the average would be needed to get close to the same mass as an oxygen atom (O) .
A) 6
B) 4
C) 12
D) 1/4
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sodium is a highly reactive metal and chlorine is a toxic gas, but when they come together the resulting material, sodium chloride (a white solid) , is essential for life. Which of the following is true when sodium and chlorine are brought into contact with one another?
A) They form a heterogeneous mixture.
B) They form a homogenous mixture
C) They form a new element.
D) They form a compound.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the atomic view of a substance shown below.  This substance would be classified as a(n)
This substance would be classified as a(n)
A) element
B) compound
C) heterogeneous mixture
D) homogeneous mixture
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the text periodic table and using the correct number of significant figures, enter the appropriate number in the blank. The mass of 7.00 mol of sodium chloride, NaCl, is _____________g.
Correct Answer

verified
Correct Answer
verified
True/False
Neutral isotopes of the same element have the same number of electrons.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the meaning of the subscripts in the formula for ethyl alcohol, C2H5OH?
A) Each formula unit contains 2 carbon atoms for each oxygen atom.
B) There are two carbon atoms per formula unit of ethyl alcohol.
C) Each formula unit contains 3 times as many hydrogen atoms as carbon atoms.
D) All of these are correct statements.
F) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The electron arrangement for Na would be: shell 1: 2 electrons shell 2: 8 electrons shell 3: 1 electron
B) False
Correct Answer

verified
Correct Answer
verified
True/False
In 22.99 g of Na there is 6.022 × 1023 atoms of Na.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 77
Related Exams