A) structural isomers
B) cis-trans isomers
C) enantiomers
D) isotopes
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the biologically important functional groups listed below, which is hydrophobic and can make cross-bridges?
A) hydroxyl
B) carboxyl
C) amino
D) sulfhydryl
E) methyl
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
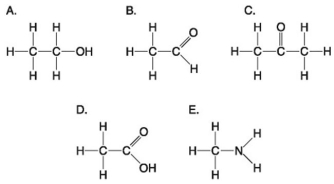 -Which molecule shown above has a carbonyl functional group in the form of a ketone?
-Which molecule shown above has a carbonyl functional group in the form of a ketone?
A) A
B) B
C) C
D) D
E) E
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which molecule(s) shown above is (are) ionized in aqueous solution at pH 7?
-Which molecule(s) shown above is (are) ionized in aqueous solution at pH 7?
A) A
B) B and D
C) D and E
D) D
E) E
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The idea that certain molecules could only be formed by living organisms was known as
A) vitalism.
B) mechanism.
C) inorganic chemistry.
D) religion.
E) isomerism.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are hydrocarbons insoluble in water?
A) The majority of their bonds are polar covalent carbon-to-hydrogen linkages.
B) The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages.
C) They are hydrophilic.
D) They exhibit considerable molecular complexity and diversity.
E) They are lighter than water.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
A) the presence or absence of bonds with oxygen atoms
B) the presence or absence of double bonds between the carbon atom and other atoms
C) the polarity of the covalent bonds between carbon and other atoms
D) the presence or absence of bonds with nitrogen atoms
E) the solvent that the organic molecule is dissolved in
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
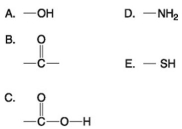 -Which functional group(s) shown above is (are) present in all amino acids?
-Which functional group(s) shown above is (are) present in all amino acids?
A) A and B
B) B and D
C) C only
D) D only
E) C and D
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Testosterone and estradiol are male and female sex hormones, respectively, in many vertebrates. In what way(s) do these molecules differ from each other?
A) Testosterone and estradiol are structural isomers but have the same molecular formula.
B) Testosterone and estradiol are cis-trans isomers but have the same molecular formula.
C) Testosterone and estradiol have different functional groups attached to the same carbon skeleton.
D) Testosterone and estradiol have distinctly different chemical structures, with one including four fused rings of carbon atoms, while the other has three rings.
E) Testosterone and estradiol are enantiomers of the same organic molecule.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organic chemistry is the study of molecules containing
A) water.
B) carbon.
C) hydrogen.
D) nitrogen.
E) oxygen.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
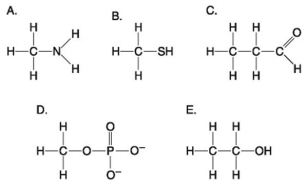 -Which molecule shown above can function as a base?
-Which molecule shown above can function as a base?
A) A
B) B
C) C
D) D
E) E
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating the differences between three organic compounds. They all have the same carbon skeleton but have differences in their functional groups. -You test for hydrophobicity and observe that one molecule type is extremely hydrophobic. Which of the following is the best possible conclusion?
A) It has no functional groups attached and is a hydrocarbon.
B) It contains at least one carboxyl group.
C) It contains an amino group.
D) It has at least one hydroxyl group.
E) It contains phosphates.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which molecule shown above can form a dimer linked by a covalent bond?
-Which molecule shown above can form a dimer linked by a covalent bond?
A) A
B) B
C) C
D) D
E) E
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which functional groups can act as acids?
A) amino and sulfhydryl
B) carbonyl and carboxyl
C) carboxyl and phosphate
D) hydroxyl and aldehyde
E) ketone and amino
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
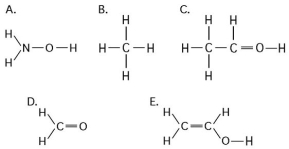 -Which of the structures illustrated above cannot form hydrogen bonds with water molecules?
-Which of the structures illustrated above cannot form hydrogen bonds with water molecules?
A) A
B) B
C) C
D) D
E) E
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which molecule shown above would have a positive charge in aqueous solution at pH 7?
-Which molecule shown above would have a positive charge in aqueous solution at pH 7?
A) A
B) B
C) C
D) D
E) E
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
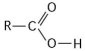 -What is the name of the functional group shown in the figure above?
-What is the name of the functional group shown in the figure above?
A) carbonyl
B) ketone
C) aldehyde
D) carboxyl
E) hydroxyl
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which molecule shown above contains an amino functional group, but is not an amino acid?
-Which molecule shown above contains an amino functional group, but is not an amino acid?
A) A
B) B
C) C
D) D
E) E
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 78 of 78
Related Exams