A) 20.9 kJ
B) 9.36 kJ
C) 10.5 kJ
D) 210 kJ
E) 1200 kJ
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which process is exothermic?
A) freezing rain drops
B) evaporating alcohol
C) defrosting frozen food
D) warming milk
E) subliming dry ice
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many joules are there in one glass of milk containing 110 Calories?
A) 4.6 × 105 J
B) 460 kJ
C) 2.6 × 104 J
D) 26 J
E) 0.46 J
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.
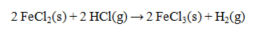
A) -219.0 kJ
B) -69.2 kJ
C) 34.6 kJ
D) 69.2 kJ
E) 219.0 kJ
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
How much energy in kilojoules is required to raise the temperature of 20.0 g of water from 22.0°C to 37.0°C? The specific heat of water = 4.184 J g-1 °C-1.
Correct Answer

verified
Correct Answer
verified
Short Answer
How much energy in kilojoules is required to vaporize a 25.0 g sample of water at 100.0°C? The heat of vaporization of water = 2260 J g-1 at 100°C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpies of formation for several substances are given below:  Calculate the DH° for the reaction below.
Calculate the DH° for the reaction below.
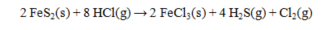
A) -881.4 kJ
B) -811.8 kJ
C) -149.6 kJ
D) +149.6 kJ
E) +213.4 kJ
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false?
A) Breaking bonds is always endothermic.
B) Bond enthalpies quantify the energy change for the complete separation of two bonded atoms in a molecule at constant pressure.
C) Bond enthalpy values are based on molecules in the gas phase.
D) Breaking weak bonds and making an equal number of strong bonds is exothermic.
E) Breaking weak bonds and making a greater number of equally weak bonds is endothermic.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The temperature of 3.50 kg of water is raised by 1.17°C when 1.00 g of hydrazine N2H4 is burned in a bomb calorimeter. The calorimeter has a heat capacity of 883 J/°C. How much heat is given off by the sample? The specific heat of water = 4.184 J g-1 °C-1.
A) 0.944 kJ
B) 16.3 kJ
C) 17.1 kJ
D) 18.2 kJ
E) 21.5 kJ
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpies of formation for several substances are given below:  Determine the DH° for the reaction below.
Determine the DH° for the reaction below.
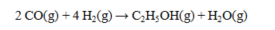
A) -366.4 kJ
B) -299.9 kJ
C) -298.5 kJ
D) -255.9 kJ
E) 255.9 kJ
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar heat capacity of aluminum (specific heat = 0.902 J g-1 °C-1) ?
A) 0.034 J mol-1 °C-1
B) 24.8 J mol-1 °C-1
C) 29.3 J mol-1 °C-1
D) 120 J mol-1 °C-1
E) 1.5 × 1025 J mol-1 °C-1
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The standard enthalpies of formation for several substances are given below:  Determine the DH° for the reaction below.
Determine the DH° for the reaction below.
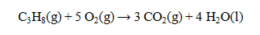
A) -2322.5 kJ
B) -2219.9 kJ
C) -782.8 kJ
D) -575.2.7 kJ
E) +575.2 kJ
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of water containing 2.00 moles is initially at 30.0°C. If the sample absorbs 2.00 kJ of heat, what is the final temperature of the water? (specific heat of water = 4.184 J g-1 °C-1)
A) 13.3°C
B) 30.2°C
C) 43.3°C
D) 46.7°C
E) 269°C
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the enthalpy change when 22.5 g of CH4 are burned in excess O2? 
A) -39.5 kJ
B) -890 kJ
C) -1250 kJ
D) +890 kJ
E) +1250 kJ
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When does an endothermic reaction occur?
A) when the enthalpy of the reactants is greater than the enthalpy of the products
B) when bonds are formed
C) when the energy of bonds breaking is greater than the energy of bonds formed
D) when the energy of bonds breaking is less than the energy of bonds formed
E) when stronger bonds are formed and weaker bonds are broken
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A bomb calorimeter has a heat capacity of 783 J/°C and contains 254 g of water. How much energy is evolved or absorbed when the temperature of the calorimeter goes from 23.73°C to 26.01°C? The specific heat of water = 4.184 J g-1 °C-1.
A) 1.78 kJ evolved
B) 2.42 kJ evolved
C) 4.21 kJ evolved
D) 2420 kJ absorbed
E) 4210 kJ evolved
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of potential energy?
A) holding a baseball
B) running around bases
C) pitching a baseball
D) swinging a bat
E) sliding into home plate
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine if each of the four processes below describes positive or negative changes to the internal energy of the system. I. water absorbs heat from the surroundings and becomes steam II. steam expands and pushes against the surrounding air III. fuel molecules burn and heat the surroundings IV. air is compressed into an inner tube by an external pump
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much heat is required to melt 125 g of ice at 0°C? The enthalpy of fusion of ice = 333 J g-1 at 0 °C.
A) 0.375 J
B) 41.6 kJ
C) 283 kJ
D) 333 J
E) 523 J
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the enthalpy change for the combustion of 4.73 g C4H10 in excess oxygen? 
A) -23200 kJ
B) -8960 kJ
C) -401 kJ
D) -154 kJ
E) -32.7 kJ
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 55
Related Exams