Correct Answer

verified
33 sigma b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the number of lone pairs around the central atom and the molecular geometry of SeF4.
A) 0 lone pairs, tetrahedral
B) 1 lone pair, distorted tetrahedron (seesaw)
C) 1 lone pair, square pyramidal
D) 1 lone pair, tetrahedral
E) 2 lone pairs, square planar
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. 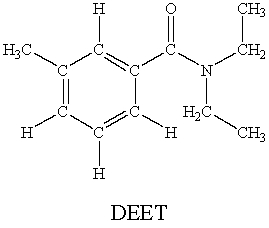 How many sigma bonds and pi bonds are contained in a DEET molecule?
How many sigma bonds and pi bonds are contained in a DEET molecule?
Correct Answer

verified
31 sigma b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The bond angle in SCl2 is expected to be
A) a little less than 109.5°.
B) 109.5°.
C) a little more than 109.5°.
D) 120°.
E) 180°.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 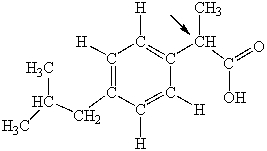
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the geometry of the ion PCl4-.
A) 0 lone pairs, tetrahedral
B) 1 lone pair, distorted tetrahedron (seesaw)
C) 1 lone pair, square pyramidal
D) 1 lone pair, tetrahedral
E) 2 lone pairs, square planar
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the type of hybrid orbitals used by the central atom in CCl4.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following would the bonding be weakened with the addition of an electron to form the negative molecular ion?
A) B2
B) C2
C) N2
D) all of these
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Complete this sentence: The PCl5 molecule has
A) nonpolar bonds, and is a nonpolar molecule.
B) nonpolar bonds, but is a polar molecule.
C) polar bonds, and is a polar molecule.
D) polar bonds, but is a nonpolar molecule.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the geometry around the central atom in PO43-.
A) trigonal planar
B) trigonal pyramidal
C) tetrahedral
D) trigonal bipyramidal
E) octahedral
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances is/are bent? (i) H2S (ii) CO2 (iii) ClNO (iv) NH2- (v) O3
A) only (iii)
B) only (i) and (v)
C) only (i) , (iii) , and (v)
D) all are bent except for (iv)
E) all are bent except for (ii)
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization on the central atom in NO3- ?
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not true of molecular orbitals?
A) The number of molecular orbitals formed is always equal to the number of atomic orbitals combined.
B) A molecular orbital can accommodate up to two electrons.
C) When electrons are added to orbitals of the same energy, the most stable arrangement is predicted by Hund's rule.
D) Low-energy molecular orbitals fill before high-energy molecular orbitals fill.
E) For any substance, the number of electrons in molecular orbitals is equal to the sum of all the valence electrons on the bonding atoms.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angle in Cl2O is expected to be approximately
A) 90°.
B) 109.5°.
C) 120°.
D) 145°.
E) 180°.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has the largest dipole moment (i.e., is the most polar) ?
A) CH4
B) CH3Br
C) CH3Cl
D) CH3F
E) CH3I
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the geometry of the ion ClO2-.
A) 0 lone pairs, linear
B) 1 lone pair, bent
C) 2 lone pairs, bent
D) 3 lone pairs, bent
E) 3 lone pairs, linear
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Draw a Lewis structure for PF5 that shows the correct atom arrangement predicted by VSEPR theory.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of lone pairs around the central atom and the geometry of the ion NO2-.
A) 0 lone pairs, linear
B) 1 lone pair, bent
C) 2 lone pair, bent
D) 3 lone pairs, bent
E) 3 lone pairs, linear
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly lists species in order of increasing bond order?
A) C2 < Li2 < Be2 < N2
B) Be2 < Li2 < C2 < N2
C) N2 < Be2 < Li2 < C2
D) N2 < C2 < Li2 < Be2
E) Be2 < C2 < N2 < Li2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 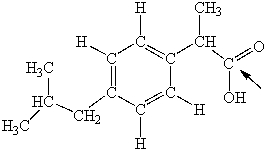
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 120
Related Exams