A) transparency
B) density
C) polarity
D) phase
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why can you determine wind direction by wetting your finger and holding it up in the air?
A) Wind carries water molecules, so you can sense the water molecules condensing on the side of your finger that faces the wind.
B) The wind hitting your finger cools the water down, slowing down evaporation. So the cooler side of your finger is windward.
C) When a wet finger is held to the wind, evaporation is greatest on the windy side, which feels cool. The cool side of your finger is windward.
D) Wind direction cannot be determined by wetting your finger because evaporation will occur evenly over the wetted finger.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the diagram above describing the phase of xenon relative to temperature and pressure what is the phase of xenon at 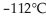 and
and 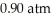 ?
?
A) solid
B) liquid
C) gas
D) melting solid
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It easier to cool off on a dry day vs. a humid day because the rate of evaporation is ________.
A) greater on a dry day and more heat is lost
B) slower on a dry day and more heat is lost
C) slower on a dry day and less heat is lost
D) greater on a dry day and less heat is lost
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does liquid water expand slightly when cooled from 4°C to 2°C?
A) The molecules vibrate faster and the molecules move farther apart.
B) The molecules vibrate faster and the molecules move closer together.
C) The molecules vibrate slower and the molecules move farther apart as hydrogen bonds form.
D) The molecules vibrate slower and the molecules move closer together as hydrogen bonds form.
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the temperature change if you heat water from 55°C to 100°C?
A) 55°C
B) 100°C
C) 45°C
D) -45°C
E) -55°C
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gold ring cannot sit on the surface of water, but a thin gold wire loop of the same diameter can. Why?
A) The gold ring applies a much greater pressure against the surface of the water so that it is able to push through the surface tension.
B) The surface area of contact is the same, but the weight of the gold ring is much greater.
C) The thin gold wire is far less dense than the gold ring, which allows it to float.
D) Both A and B are true.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A water bug can rest on top of water without getting wet because of ________.
A) surface tension
B) cohesive forces within water
C) its nonpolar exterior
D) all of the above
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does soap decrease the surface tension of of water?
A) It keeps water molecules away from the surface.
B) It increases the capillary action of the water.
C) It increases the ionic content of the water.
D) It decreases the amount of intermolecular attraction between the water molecules.
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes the usual properties of most compounds besides water?
A) The density of a material increases when it solidifies.
B) The volume of a material increases when it solidifies.
C) The mass of a material increases when it solidifies.
D) The molecules become more closely packed when they liquify.
E) none of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Where is the boiling point of water the greatest: at the bottom of a pot of water or just below the surface?
A) The boiling point of water is the same throughout the pot.
B) The boiling point of water is is the greatest at the bottom where the density is greatest.
C) The boiling point of water is greatest where the water pressure is least, which is just below the surface.
D) The boiling point of water is greatest where the water pressure is greatest, which is at the bottom.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Condensation is ________.
A) always a cooling process
B) sometimes a cooling process
C) always a warming process
D) sometimes a warming process
E) Two of the above are correct.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When you increase the temperature of a substance, the molecules vibrate ________.
A) faster and the molecules move farther apart
B) faster and the molecules more closer together
C) slower and the molecules move farther apart
D) slower and the molecules move closer together
E) none of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ice is slippery because ________.
A) air molecules have been excluded
B) of greater kinetic energy at the surface
C) of its relatively high purity
D) surface crystals fall apart
E) of its tight internal and external structuring
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A cohesive force is best described as the ________.
A) attraction between two liquid molecules
B) attraction between a liquid molecule and a solute
C) force that allows for molecular cohesion
D) force that holds together the nucleus
E) attraction between molecules of two different substances
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains the least heat energy?
A) 1 gram of steam at 100°C
B) 1 gram of water at 100°C
C) 1 gram of water at 50°C
D) 1 gram of water at 0°C
E) 1 gram of ice at 0°C
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Bermuda is close to North Carolina, but unlike North Carolina it has a tropical climate year round. Why is this so?
A) Since North Carolina is so humid, much of the sun's energy is absorbed by the water in the air, which has a high heat capacity.
B) North Carolina has a much higher elevation than sea level, keeping the state cool.
C) Since Bermuda is surrounded by water, its temperatures are moderated by the high specific heat of water. When the air is cooler than the water, the water warms the air; when the air is warmer than the water, the water cools the air.
D) The winds blow from the west, cooling North Carolina, but they blow from the east over Bermuda, which causes the tropical climate of that island.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Your instructor hands you a closed flask of room-temperature water. When you hold it, the heat from your bare hands causes the water to boil. Quite impressive! How is this accomplished?
A) The pressure inside the flask is very high, so only a slight increase in temperature is required to bring the water to a boil.
B) Your instructor has tricked you and there must be a liquid, such as alcohol, with a lower boiling point than water.
C) A salt must be dissolved in the water to lower its boiling point.
D) The air in the flask is very low in pressure, so that the heat from your hand (not the pressure from your hand) will produce boiling at this reduced pressure.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Is the density of near-freezing water, which contains microscopic ice crystals, greater or less than the density of liquid water containing no microscopic ice crystals?
A) less dense
B) more dense
C) It's the same since it's all water.
D) It's not possible to determine the density of any substance which exists in two phases at once.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does blowing over hot soup cool the soup?
A) When you blow over the top of a bowl of hot soup, you remove the warm vapor which tends to condense and reduce net evaporation above the bowl
B) When you blow over the top of a bowl of hot soup, the moving air reduces pressure atop the soup and increases the rate of evaporation.
C) When you blow over the top of a bowl of hot soup, you increase net evaporation.
D) all of the above
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 140
Related Exams