A) 4.2 × 10-2
B) 8.8 × 10-2
C) 65
D) 77
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equilibrium mixture of CO,O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0020 M O2.At this temperature,Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) . What is the equilibrium concentration of CO?
A) 3.6 × 10-6 M
B) 1.9 × 10-3 M
C) 7.0 × 10-2 M
D) 2.6 × 10-1 M
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the equilibrium equation for the following reaction? FeS(s) + 2 H3O+ (aq) ⇌ Fe2+(aq) + H2S (aq) + 2 H2O (l)
A) Kc = ![]()
B) Kc = ![]()
C) Kc = ![]()
D) Kc = ![]()
F) All of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
The reaction below is heated from 20°C to 91°C,according to Le Châtelier's principle,there will be a net reaction from ________ to ________,and the brown color will become ________. N2O4(g)⇌ 2 NO2(g)ΔH°= + 57.2 kJ colorless brown
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium equation is also known as the law of
A) coefficients.
B) constant concentration.
C) dynamic equilibrium.
D) mass action.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant Kc for the reaction HF(aq) + H2O(l) ⇌ H3O+(aq) +F-(aq) is 3.5 × 10-4.What is the equilibrium concentration of H3O+ if the initial concentration of HF is 1.0 M?
A) 1.0 M
B) 3.5 × 10-2 M
C) 1.9 × 10-2 M
D) 1.9 × 10-4 M
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An equilibrium mixture of CO,O2 and CO2 at a certain temperature contains 0.0010 M CO2 and 0.0015 M O2.At this temperature,Kc,equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) . What is the equilibrium concentration of CO?
A) 4.8 × 10-6 M
B) 2.2 × 10-3 M
C) 9.3 × 10-2 M
D) 3.1 × 10-1 M
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For acid solutions of the same molarity acid strength is proportional to the equilibrium concentration of H3O+.For equimolar solutions of acids,which equilibrium expression below corresponds to the strongest acid?
A) Kc = ![]()
= 3.5 × 10-4
B) Kc = ![]()
= 3.5 × 10-8
C) Kc = ![]()
= 4.5 × 10-4
D) Kc = ![]()
= 4.9 × 10-10
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
At 1000 K,Kp = 19.9 for the reaction Fe2O3(s)+ 3 CO(g)⇌ 2 Fe(s)+ 3 CO2(g).What is the value of Kp for the reaction 8 Fe(s)+ 12 CO2(g)⇌ 4 Fe2O3(s)+ 12 CO(g)?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about the equilibrium constant is true? The value of Kc
A) changes as product concentration changes.
B) changes as reactant concentration changes.
C) changes as temperature changes.
D) never changes.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the isomerization reaction: butane ⇌ isobutane Kp equals 25 at 500°C.If the initial pressures of butane and isobutane are 10.atm and 0.0 atm,respectively,what are the pressures of the two gases at equilibrium?
A) P(butane) = 0.38 atm and P(isobutane) = 9.6 atm
B) P(butane) = 0.40 atm and P(isobutane) = 10.atm
C) P(butane) = 9.6 atm and P(isobutane) = 0.38 atm
D) P(butane) = 10 atm and P(isobutane) = 0.40 atm
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent mixtures of cis-C2H2X2 molecules and trans-C2H2X2 molecules,which interconvert according to the equation cis-C2H2X2 ⇌ trans-C2H2X2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 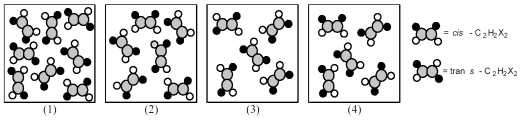
A) mixture (2)
B) mixture (3)
C) mixture (4)
D) None of the other mixtures are at equilibrium.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following picture represents the equilibrium state for the reaction A2 + B2 ⇌ 2AB.What is the relationship between the rate constant for the forward reaction,kf,and the rate constant for the reverse reaction kr? 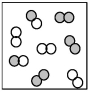
A) kf < kr
B) kf = kr = 0
C) kf = kr
D) kf > kr
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction HCO3- (aq) + H2O (l) ⇌ CO3-2 (aq) + H3O+ (aq) The Keq for this reaction is 5.6 × 10-11.Describe what will happen to the reaction if the concentration of each reactant is [HCO3-] = 5.6 × 10-11 [H3O+] = 1.2 × 10-11 [CO3-] = 5.6 × 10-11
A) Reaction will shift right,concentration of products will increase.
B) Reaction will shift left,concentration of reactants will increase.
C) Reaction will not change,it is at equilibrium.
D) Not enough information to determine the answer.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitric oxide reacts with oxygen to form nitrogen dioxide: 2 NO(g) + O2(g) ⇌ 2 NO2(g) What is Kc' for the reverse reaction if the equilibrium concentration of NO is 0.300 M,O2 is 0.200 M,and NO2 is 0.530 M at 25°C?
A) 0.0340
B) 0.0641
C) 0.624
D) 15.6
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 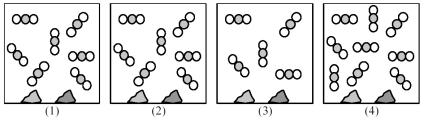 -For the reaction: N2(g) + 2 O2(g) ⇌ 2 NO2(g) ,Kc = 8.3 × 10-10 at 25°C.What is the concentration of N2 gas at equilibrium when the concentration of NO2 is twice the concentration of O2 gas?
-For the reaction: N2(g) + 2 O2(g) ⇌ 2 NO2(g) ,Kc = 8.3 × 10-10 at 25°C.What is the concentration of N2 gas at equilibrium when the concentration of NO2 is twice the concentration of O2 gas?
A) 2.1 × 10-10 M
B) 4.2 × 10-10 M
C) 2.4 × 109 M
D) 4.8 × 109 M
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements does not describe the equilibrium state?
A) Equilibrium is dynamic and there is no net conversion to reactants and products.
B) The concentration of the reactants is equal to the concentration of the products.
C) The concentration of the reactants and products reach a constant level.
D) The rate of the forward reaction is equal to the rate of the reverse reaction.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a certain temperature,Kc equals 1.4 × 102 for the reaction: 2 CO(g) + O2(g) ⇌ 2 CO2(g) . If a 5.00-L flask contains 0.400 mol of CO2 and 0.100 mol of O2 at equilibrium,how many moles of CO are also present in the flask?
A) 1.20 mol
B) 0.239 mol
C) 0.107 mol
D) 0.0114 mol
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium carbonate is relatively insoluble and the dissolution reaction is endothermic: CaCO3(s) ⇌ Ca2+(aq) + CO32-(aq) . Which change in reaction condition below will shift the equilibrium to the right?
A) add an acid to react with CO32- ion
B) add an anion with which Ca2+ is even less soluble than calcium carbonate
C) increase the temperature
D) All of these will shift reaction to the right.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If Kc is the equilibrium constant for a forward reaction what is Kc' for the reverse reaction?
A) - Kc
B) Kc
C) (Kc) -1
D) none of these
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 171
Related Exams