A) boron trifluoride.
B) triboron fluoride.
C) tri (bromine fluoride) .
D) tribromine fluoride.
E) bromine trifluoride.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element listed is the least electronegative?
A) nitrogen
B) hydrogen
C) oxygen
D) fluorine
E) chlorine
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of valence electrons in the acetic acid molecule (CH3CO2H) is ________.
A) 0
B) 8
C) 16
D) 24
E) 32
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed when two atoms share three pairs of electrons is a ________ bond; it is best described as ________.
A) double; covalent
B) single; covalent
C) triple; covalent
D) double; ionic
E) triple; ionic
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A section of the Periodic Table containing main group elements is shown.Which bond would be least polar? 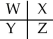
A) WX
B) YZ
C) WZ
D) WY
E) cannot be determined without more specific information
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The covalent bonding model is most useful in describing which of the following compounds?
A) CoCl2
B) MgCl2
C) NiCl2
D) SCl2
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for sulfur hexabromide is ________.
A) SF6
B) SBr6
C) SBr4
D) SiBr6
E) SiB6
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule that contains three identical polar bonds to the central atom will be ________.
A) nonpolar if the geometry is planar triangular
B) polar in all cases
C) nonpolar in all cases
D) impossible to tell the polarity
E) either polar or nonpolar depending on the identity of the atoms bonded to the central atom
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Covalent compounds form distinct molecules,and therefore may exist as gases,liquids,or solids at room temperature,depending on the characteristics of the compound.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed when two atoms share one pair of electrons is a ________ bond; it is best described as ________.
A) double; covalent
B) double; ionic
C) single; covalent
D) single; ionic
E) triple; covalent
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for CCl4.What is the molecular geometry of this compound? Is the molecule polar or nonpolar?
Correct Answer

verified
 tetrahedr...
tetrahedr...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Match the following. -phosphorus trichloride
A) OF2
B) BrF5
C) CCI4
D) N2O4
E) SF6
F) CO
G) ICl
H) SO2
I) CH4
J) IF7
K) PCI3
L) NH3
N) E) and H)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which representation of a hydrogen molecule is not correct?
A) H=H
B) H2
C) H:H
D) HH
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A triple bond involves the sharing of ________ pairs of electrons between the atoms
A) 1
B) 2
C) 3
D) 4
E) 5
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule SiCl4.The electronegativity values for Si and Cl are 1.8 and 3.0,respectively.Based on these values and on consideration of molecular geometry,the Si-Cl bond is ________ and the molecule is ________.
A) polar; polar
B) nonpolar; nonpolar
C) polar; nonpolar
D) nonpolar; polar
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 75 of 75
Related Exams