A) [He]
B) [Kr]
C) [Ar]
D) [Ne]
E) [Rn]
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements would be expected to have chemical and physical properties most similar to those of the barium (Ba) ?
A) magnesium (Mg)
B) argon (Ar)
C) aluminum (Al)
D) tin (Sn)
E) potassium (K)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What 2+ ion has the following ground state electron configuration? 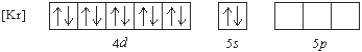
A) Cd2+
B) Sr2+
C) Zn2+
D) Sn2+
E) None
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following ions in order from smallest to largest ionic radii: K+,Na+,Mg2+,and Al3+.
A) Al3+ < Mg2+ < Na+ < K+
B) Na+ < Mg2+ < Al3+ < K+
C) K+ < Mg2+ < Na+ < Al3+
D) K+ < Al3+ < Mg2+ < Na+
E) Mg2+ < Al3+ < Na+ < K+
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons can be described by the following quantum numbers: n = 6, 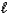 = 4,
= 4, 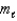 = -4?
= -4?
A) 1
B) 2
C) 6
D) 10
E) 18
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electron configurations corresponds to the ground state of an atom of a transition element?
A) 1s22s22p3
B) 1s22s22p63s23p63d104s24p1
C) 1s22s22p63s23p63d24s2
D) 1s22s22p63s23p64s2
E) 1s22s22p63s23p4
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
________ rule states that the most stable arrangement of electrons is that which contains the maximum number of unpaired electrons,all with the same spin direction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A metal oxide forms when strontium reacts with oxygen.What is the most likely formula of this metal oxide?
A) SrO
B) Sr2O
C) Sr2O3
D) SrO2
E) SrO3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Electron ________ is defined as the energy change for a process in which a gas phase atom acquires an electron.
Correct Answer

verified
affinity
Correct Answer
verified
Multiple Choice
Which of the following has the same (total) number of electrons as Kr?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following atoms in order decreasing atomic radii: C,N,B,Al.
A) Al > B > C > N
B) B > Al > C > N
C) C > B > Al > N
D) B > C > N > Al
E) Al > C > B > N
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ions have the same ground state electron configuration: S2-,N3-,Mg2+,and Br-?
A) N3- and Mg2+
B) S2-,N3-,and Br-
C) S2- and Br-
D) Mg2+ and Br-
E) S2-,N3-,Mg2+,and Br-
G) None of the above
Correct Answer

verified
A
Correct Answer
verified
Short Answer
The f-block elements are also referred to as the ________ and actinides.
Correct Answer

verified
lanthanides
Correct Answer
verified
Multiple Choice
Which of the following electron configurations is not allowed?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In general,atomic radii
A) decrease down a group and remain constant across a period.
B) decrease down a group and increase across a period.
C) increase down a group and increase across a period.
D) increase down a group and remain constant across a period.
E) increase down a group and decrease across a period.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground state electron configuration for Cr3+?
A) [Ar]
B) [Ar]3d74s2
C) [Ar]3d14s2
D) [Ar]3d24s1
E) [Ar]3d3
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which elements have no affinity for electrons?
A) transition metals
B) s-block elements
C) main group nonmetals
D) noble gases
E) semiconductors
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A metal nitride forms when sodium reacts with elemental nitrogen.What is the most likely formula of this metal nitride?
A) NaN
B) Na3N
C) Na2N3
D) Na3N2
E) NaN3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of which of the following elements has the smallest atomic radius?
A) F
B) Rb
C) Ca
D) Ge
E) P
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Essay
Explain why the first ionization energy for oxygen is lower than that for nitrogen.
Correct Answer

verified
Oxygen has a 1s22s22p4 electron configurati...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 1 - 20 of 82
Related Exams