A) 1.63 L
B) 0.154 L
C) 6.50 L
D) 0.615 L
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the volume of 28.0 g of nitrogen gas at STP?
A) 33.6 L
B) 11.2 L
C) 22.4 L
D) 44.8 L
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final volume (L) of 10.0 L system that has the pressure quartered?
A) 0.250
B) 17.1
C) 2.50
D) 40.0
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the temperature (°C) of 2.48 moles of gas stored in a 30.0 L container at 1559 mm Hg? (R= 0.0821 L atm/ mol K)
A) 302
B) 189
C) 29
D) -84
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The unit of pressure known as the atmosphere (atm)is defined as the average pressure found at the top of Mount Everest.
B) False
Correct Answer

verified
False
Correct Answer
verified
True/False
For all gas law calculations,the temperature must be in kelvins.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Gas particles act independently of each other.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Gas particles lose energy every time they collide with each other or the container wall.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is impossible for an ideal gas?
A) ![]()
=
![]()
(
![]()
)
B) ![]()
=
![]()
C) V2 = ( ![]()
) V1
D) V1T1 = V2T2
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The volume of 9.00 grams of water vapor at STP is 11.2 L.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Dalton's law of partial pressure is: P = 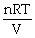 .
.
B) False
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
Which of following statements are consistent with the Kinetic Molecular Theory?
A) Gases are compressible because the volume of atoms is almost entirely open space.
B) Gases assume the shape and volume of their container because they are in constant,straight-line motion.
C) Gases have a low density because there is so much empty space between the particles.
D) Gas particles collide with each other and surfaces without losing any energy.
E) All of the above statements are consistent with the Kinetic Molecular Theory.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the change in temperature of a 2.50 L system when it is compressed to 1.75 L if the initial temperature was 298 K?
A) 209 K
B) 290 K
C) -89 K
D) -98 K
E) none of the above
G) A) and E)
Correct Answer

verified
C
Correct Answer
verified
True/False
A pedometer is a device created by Torricelli to measure pressure.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
"Molar Mass" can be calculated using the formula: Molar mass = 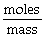 .
.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final pressure of a system (atm) that has the volume increased from 0.75 L to 1.1 L with an initial pressure of 1.25 atm?
A) 1.1
B) 0.85
C) 1.8
D) 1.2
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
To solve problems using Boyle's Law,which mathematical equation should be used?
A) ![]()
=
![]()
B) P1V1 = P2V2
C) ![]()
=
![]()
D) P2V1 = P1V2
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ammonia gas decomposes according to the equation: 2NH3(g) → N2(g) + 3H2(g) If 15.0 L of nitrogen is formed at STP,how many liters of hydrogen will be produced (also measured at STP) ?
A) 15.0 L
B) 30.0 L
C) 45.0 L
D) 90.0 L
E) not enough information
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which set of conditions reflect STP?
A) 298 K,1 atm
B) 25°C,14.7 psi
C) 373 K,760 torr
D) 273 K,1 Pa
E) 273 K,760 mm Hg
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The vapor pressure of water is independent of temperature.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 112
Related Exams