A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following hydrogen atoms from highest to lowest in acidity in the structure shown below. 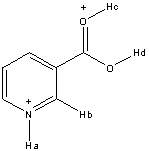
A) Ha > Hb > Hc > Hd
B) Ha > Hc > Hd > Hb
C) Hc > Hd > Ha > Hb
D) Hd > Ha > Hc > Hb
E) Hc > Ha > Hd > Hb
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 
A) V > III > II > IV > I
B) II > V > III > I > IV
C) V > III > II > I > IV
D) III > I > V > II > IV
E) III > V > II > I > IV
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which base would not effectively deprotonate phenol (PhOH) ?
A) i-PrMgBr
B) NaHCO3
C) NaH
D) (i-Pr) 2NLi
E) t-BuOK
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Protons or other electron-deficient centers that seek out electron-rich molecules are known as ___.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is/are the product(s) of the following acid-base mechanism? 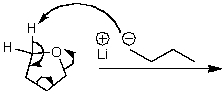
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of these choices.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction,which statement is true taking S into consideration? 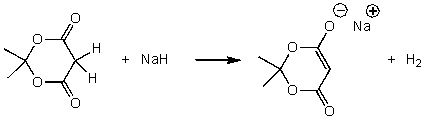
A) The reaction is an exothermic reaction and S is positive.
B) The reaction is an exothermic reaction and S is negative.
C) The reaction is an endothermic reaction and S is positive.
D) The reaction is an endothermic reaction and S is approximately zero.
E) None of these choices.
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the reaction between the following two species produce? 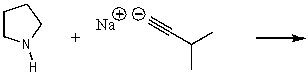
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) No reaction
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Adding sodium hydride to ethanol would produce:
A) CH3CH2OCH2CH3 + H2
B) CH3CH2OCH2CH3 + NaOH
C) CH3CH2ONa + H2
D) CH3CH2Na + NaOH
E) CH3CH3 + NaOH
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which might be used as a protic solvent?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these phosphorus-based acids is diprotic?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 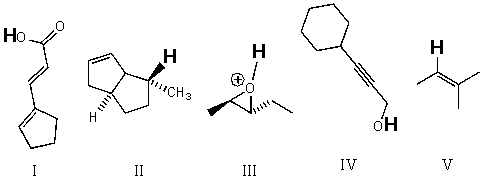
A) III > IV > I > V > II
B) II > III > V > IV > I
C) II > V > III > IV > I
D) V > III > II > IV > I
E) III > I > IV > V > II
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 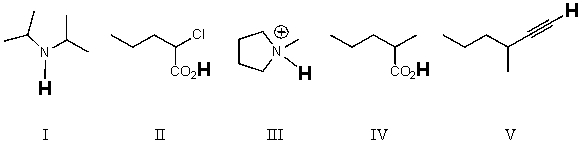
A) II > IV > V > III > I
B) IV > II > III > V > I
C) V > III > IV > II > I
D) II > IV > III > V > I
E) IV > II > I > V > III
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an untrue statement?
A) The % dissociation of a weak acid increases with increasing dilution of the acid solution.
B) The stronger an acid,the weaker its conjugate base.
C) The larger the value of Ka for an acid,the smaller the value of its pKa.
D) Comparison of the acidity of strong acids in solution requires the use of a solvent less basic than water.
E) The stronger the acid,the more positive the value of Gº for the dissociation.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction,Na+NH2- + CH3OH CH3O-Na+ + NH3,the stronger base is:
A) NaNH2
B) CH3OH
C) CH3ONa
D) NH3
E) This is not an acid-base reaction.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Carboxylic acids have conjugate bases that are resonance stabilized,which typically makes them much more acidic than alcohols.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
It is impossible to have pKa values of organic acids that are negative.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the simple hydrides,MHn,pKa values decrease in the order:
A) CH4 > NH3 > H2O > H2S > HBr
B) HBr > H2S > H2O > NH3 > CH4
C) HBr > H2O > NH3 > H2S > CH4
D) NH3 > H2S > CH4 > H2O > HBr
E) H2S > H2O > HBr > NH3 > CH4
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
This species is a carbon-based Lewis acid:
A) CH4
B) HCCl3
C) CH3+
D) :CH3-
E) ·CH3
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Adding sodium amide (NaNH2) to 1-butyne (CH3CH2C≡CH) would produce:
A) CH3CH2C≡CNa + NH3
B) CH3CH2C≡C-C≡CH2CH3 + NaH + NH3
C) CH3CH2C≡CNH2 + NaH
D) NaCH2CH2C≡CH + NH3
E) CH3CH(Na) C≡CH + NH3
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 149
Related Exams