A) Blood pH will decrease slightly.
B) Blood pH will increase slightly.
C) Blood pH will remain unchanged.
D) Blood pH will first increase, then decrease as CO₂ combines with hemoglobin.
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen (N) is much more electronegative than hydrogen (H) . Which of the following statements about the atoms in ammonia (NH3) is correct?
A) Each hydrogen atom has a partial positive charge; the nitrogen atom has a partial negative charge.
B) The nitrogen atom has a full positive charge; each hydrogen atom has a full positive charge.
C) Each hydrogen atom has a partial negative charge; the nitrogen atom has a full positive charge.
D) The nitrogen atom has a partial positive charge; each hydrogen atom has a partial negative charge.
E) There are nonpolar covalent bonds between the hydrogen atoms and polar covalent bonds between each hydrogen atom and the nitrogen atom.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a salamander clings to surfaces through hydrogen bonds, it would have the most difficulty clinging to which of the following surfaces?
A) a surface coated with a thin film of water
B) a surface coated with a thin film of vinegar (acetic acid)
C) a surface coated with a thin film of vegetable oil
D) a surface coated with a thin film of ammonia (NH3)
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have two beakers. One contains a solution of HCl at pH = 1.0. The other contains a solution of NaOH at pH = 13. Into a third beaker, you slowly and cautiously pour 20 mL of the HCl and 20 mL of the NaOH. After complete stirring, the pH of the mixture will be
A) 2.0.
B) 12.0.
C) 7.0.
D) 5.0.
E) 9.0.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Glucose has a molecular mass of 180 g/mol. How many glucose molecules are present in 90 grams of glucose?
A) 90 × 1023
B) (6.02/180) × 1023
C) (6.02/90) × 1023
D) (90 × 6.02) × 1023
E) (90/180) × 6.02 × 1023
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following takes place as an ice cube cools a drink?
A) Molecular collisions in the drink increase.
B) Kinetic energy in the drink decreases.
C) A calorie of heat energy is transferred from the ice to the water of the drink.
D) The specific heat of the water in the drink decreases.
E) Evaporation of the water in the drink increases.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
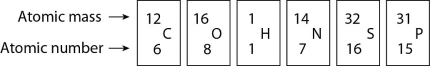 Figure 2.4
-Based on electron configuration, which of the elements in Figure 2.4 would exhibit a chemical behavior most like that of oxygen?
Figure 2.4
-Based on electron configuration, which of the elements in Figure 2.4 would exhibit a chemical behavior most like that of oxygen?
A) carbon
B) hydrogen
C) nitrogen
D) sulfur
E) phosphorus
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
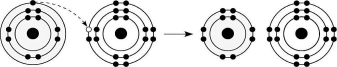 Figure 2.6
-
Figure 2.6
-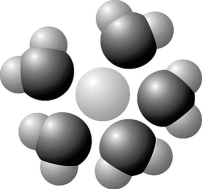 Figure 2.7
Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.7 is most likely
Figure 2.7
Based on your knowledge of the polarity of water molecules, the solute molecule depicted in Figure 2.7 is most likely
A) positively charged.
B) negatively charged.
C) without charge.
D) hydrophobic.
E) nonpolar.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does ice float in liquid water?
A) The high surface tension of liquid water keeps the ice on top.
B) The ionic bonds between the molecules in ice prevent the ice from sinking.
C) Ice always has air bubbles that keep it afloat.
D) Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water.
E) The crystalline lattice of ice causes it to be denser than liquid water.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the cytoplasm of a cell is at pH 7, and the mitochondrial matrix is at pH 8, this means that
A) the concentration of hydrogen ions is tenfold higher in the cytoplasm than in the mitochondrial matrix.
B) the concentration of hydrogen ions is tenfold higher in the mitochondrial matrix than in the cytoplasm.
C) the concentration of hydrogen ions in the cytoplasm is 7/8 the concentration in the mitochondrial matrix.
D) the mitochondrial matrix is more acidic than the cytoplasm.
E) the concentration of hydrogen ions in the cytoplasm is 8/7 the concentration in the mitochondrial matrix.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are the strongest molecular interactions?
A) van der Waals interactions
B) van der Waals interactions in a nonpolar environment
C) ionic bonds in an aqueous environment
D) covalent bonds
E) hydrogen bonds
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Approximately what percentage of human-generated atmospheric CO₂ is absorbed by the oceans?
A) 1%
B) 5%
C) 25%
D) 60%
E) 95%
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
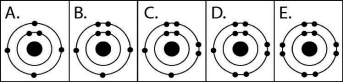 Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
Figure 2.3
-Which drawing in Figure 2.3 depicts the electron configuration of an atom capable of forming three covalent bonds with other atoms?
A) A
B) B
C) C
D) D
E) E
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of hydrogen atoms that can be covalently bonded in a molecule containing two carbon atoms?
A) 2
B) 3
C) 4
D) 6
E) 8
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar mass of glucose (C₆H₁₂O₆) is 180 g/mol. Which of the following procedures should you carry out to make a 0.5 M solution of glucose?
A) Dissolve 0.5 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
B) Dissolve 90 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
C) Dissolve 180 g of glucose in a small volume of water, and then add more water until the total volume of the solution is 1 L.
D) Dissolve 0.5 g of glucose in 1 L of water.
E) Dissolve 180 g of glucose in 0.5 L of water.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? C₆H₁₂O₆ → ________ C2H6O + ________ CO₂
A) 1; 2
B) 3; 1
C) 1; 3
D) 2; 2
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electron pairs are shared between carbon atoms in a molecule that has the formula C2H6?
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes chemical equilibrium?
A) Forward and reverse reactions continue with no effect on the concentrations of the reactants and products.
B) The concentrations of the products are higher than the concentrations of the reactants.
C) Forward and reverse reactions have stopped so that the concentrations of the reactants and products remain constant.
D) Reactions stop only when all reactants have been converted to products.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many glucose molecules are contained in one liter of a 0.1 M solution of glucose in water?
A) 6.02 × 1023
B) 3.01 × 1023
C) 6.02 × 1024
D) 12.04 × 1023
E) 6.02 × 1022
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrophobic substances such as vegetable oil are
A) nonpolar substances that repel water molecules.
B) nonpolar substances that have an attraction for water molecules.
C) polar substances that repel water molecules.
D) polar substances that have an affinity for water.
E) charged molecules that hydrogen-bond with water molecules.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 137
Related Exams