A) Living organisms do not obey the second law of thermodynamics, which states that the entropy of an organism increases with each energy transformation.
B) The decrease in entropy is associated with growth of an organism. As a consequence of growth, organisms cause a greater increase in entropy in their environment than the decrease in entropy associated with their increased complexity.
C) As a consequence of growth, the decrease in entropy of the organism is associated with a corresponding decrease in the entropy of the universe.
D) Living organisms are able to transform chemical energy into entropy.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of potential rather than kinetic energy?
A) Water rushing over Niagara Falls.
B) Light flashes emitted by a firefly.
C) A molecule of glucose.
D) A crawling beetle foraging for food.
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the thermodynamic barrier that must be overcome before products are formed in a spontaneous reaction?
A) entropy
B) activation energy
C) the equilibrium point
D) free energy
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following graphs most likely describes the effect of pH on the function of the enzyme catalase in human cells? Note: The x-axis is pH and the y-axis is enzyme activity.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes the first law of thermodynamics?
A) Energy cannot be created or destroyed.
B) The entropy of the universe is decreasing.
C) The entropy of the universe is constant.
D) Energy cannot be transferred or transformed.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When ATP releases some energy, it also releases inorganic phosphate. What happens to the inorganic phosphate in the cell?
A) It is secreted as waste.
B) It is used only to regenerate more ATP.
C) It may be used to form a phosphorylated intermediate.
D) It enters the nucleus to be incorporated in a nucleotide.
F) A) and C)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A chemical reaction that has a positive ΔG is best described as ________.
A) endergonic
B) enthalpic
C) spontaneous
D) exergonic
F) A) and B)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Why do hydrolysis reactions occur more readily in solution than dehydration reactions?
A) Hydrolysis reactions increase G, or Gibbs free energy of the system.
B) Hydrolysis reactions are endergonic and increase entropy of the system.
C) Hydrolysis reactions are exergonic and decrease entropy of the system.
D) Hydrolysis reactions are exergonic and increase entropy of the system.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does a noncompetitive inhibitor decrease the rate of an enzyme-catalysed reaction?
A) by binding to the active site of the enzyme, thus preventing binding of the normal substrate
B) by binding to an allosteric site, thus changing the shape of the active site of the enzyme
C) by decreasing the free-energy change of the reaction catalysed by the enzyme
D) by binding to the substrate, thus changing its shape so that it no longer binds to the active site of the enzyme
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
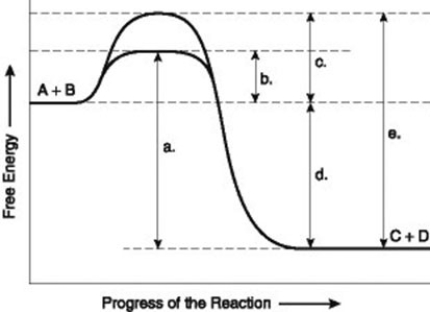 -The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following represents the activation energy required for the non-enzyme-catalysed reaction in the figure?
-The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following represents the activation energy required for the non-enzyme-catalysed reaction in the figure?
A) a
B) b
C) c
D) d
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes an example of cooperativity associated with enzyme regulation?
A) binding of the end product of a metabolic pathway to the first enzyme in the pathway to inhibit the enzyme
B) one enzyme in a metabolic pathway passing its product to act as a substrate for the next enzyme in the pathway
C) binding a substrate to one subunit of a tetramer stimulates faster binding of substrate to each of the other three subunits
D) binding of an ATP molecule along with another substrate molecule in the active site of the enzyme
F) A) and B)
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The mathematical expression for the change in free energy of a system is ΔG = ΔH - TΔS. Which of the following statements is correct?
A) ΔS is the change in enthalpy, a measure of randomness.
B) ΔH is the change in entropy, the energy available to do work.
C) ΔG is the change in free energy.
D) T is the temperature in degrees Celsius.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is a logical consequence of the second law of thermodynamics?
A) If the entropy of a system increases, there must be a corresponding decrease in the entropy of the universe.
B) If the entropy of a system decreases, there must be a corresponding decrease in the entropy of the universe.
C) If there is an increase in the energy of a system, there must be a corresponding decrease in the energy of the rest of the universe.
D) Each chemical reaction in an organism must increase the total entropy of the universe.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following terms most precisely describes the cellular process of breaking down large molecules into smaller ones?
A) catabolism (catabolic pathways)
B) metabolism
C) anabolism (anabolic pathways)
D) dehydration
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is consistent with the second law of thermodynamics?
A) A gain of free energy in a system is always associated with conversion of energy from one form to another.
B) A constant input of energy is required to maintain the high level of cellular organisation.
C) Without an input of energy, the entropy of an organism would tend to decrease over time.
D) Every energy transformation performed by an organism decreases the entropy of the universe.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the evolution of life on Earth, from simple prokaryote-like cells to multicellular eukaryotic organisms, is true?
A) By resulting in such diversity and complexity of life, it is an exception to the second law of thermodynamics.
B) It has occurred in accordance with the laws of thermodynamics and resulted in a substantial increase in the entropy of the planet.
C) It has occurred in accordance with the laws of thermodynamics and resulted in a substantial increase in the total energy in the universe.
D) It has occurred in accordance with the laws of thermodynamics and resulted in a substantial decrease in the entropy of the planet.
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A series of enzymes catalyse the reactions in the metabolic pathway X → Y → Z → A. Product A binds to the enzyme that converts X to Y at a position remote from its active site. This binding decreases the activity of the enzyme. With respect to the enzyme that converts X to Y, substance A functions as ________.
A) an allosteric inhibitor
B) the substrate
C) an intermediate
D) a competitive inhibitor
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
During a laboratory experiment, you discover that an enzyme-catalysed reaction has a ∆G of -20 kcal/mol. If you double the amount of enzyme in the reaction, what will be the ∆G for the new reaction?
A) -40 kcal/mol
B) -20 kcal/mol
C) -10 kcal/mol
D) +20 kcal/mol
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of reactions would decrease the entropy within a cell?
A) anabolic reactions
B) hydrolysis
C) digestion
D) catabolic reactions
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chemical equilibrium is relatively rare in living cells because metabolic pathways are interconnected. Which of the following statements describes an example of a reaction that may be at chemical equilibrium in a cell?
A) An exergonic reaction in which the free energy at equilibrium is higher than the energy content of the reaction at any point away from equilibrium.
B) An exergonic reaction in which the entropy change in the cell is precisely balanced by an opposite entropy change in the cell's surroundings.
C) A chemical reaction in which neither the reactants nor the products are being produced or consumed in any metabolic pathway at that time in the cell.
D) An endergonic reaction in an active metabolic pathway where the energy for that reaction is supplied only by heat from the environment.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 67
Related Exams