A) a triple bond between the nitrogens.
B) three unpaired electrons.
C) a double bond between the nitrogens.
D) a single bond between the nitrogens.
E) none of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Refer to the SeF4 molecule. -What is the electron arrangement around the central atom?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following electronegativity values to answer the question: C 2.5 Cl 3.2 H 2.2 N 3.0 O 3.4 This molecule shows the smallest number of lone pairs in its Lewis structure.
A) CH3CHO
B) CO2
C) CH3Cl
D) C2H6
E) none of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the compound crotonaldehyde, whose skeleton is 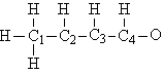 Which carbon in this molecule has tetrahedral bonding?
Which carbon in this molecule has tetrahedral bonding?
A) 2
B) 3
C) 4
D) 1
E) All have tetrahedral bonding.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion violates the octet rule?
A) I3-
B) PF3
C) H2O
D) NO3-
E) none of these
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for IF6+.
A) tetrahedral
B) pyramidal
C) octahedral
D) square planar
E) none of these
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a dipole moment?
A) PCl3
B) BCl3
C) Cl2
D) SiCl4
E) none of these
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
For each of the following compounds: -SF4 A)Give the shape of the molecule. B)Indicate the polarity of the molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the Lewis structure most like that of CO32-?
A) SO32-
B) CO2
C) NO3-
D) NO2
E) O3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angles about the carbon atom in the formaldehyde molecule, H2C = O, are about
A) 109°.
B) 60°.
C) 120°.
D) 90°.
E) 180°.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has an incomplete octet in its Lewis structure?
A) ICl
B) NO
C) Cl2
D) OF2
E) SO2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for SF5+.
A) square planar
B) octahedral
C) tetrahedral
D) pyramidal
E) none of these
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction between magnesium and sulfur, the magnesium atoms
A) share electrons with sulfur.
B) become part of polyatomic ions.
C) become anions.
D) become cations.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is correct?
A) H2O is linear.
B) The molecule ClO2 cannot be accurately described by a Lewis structure consistent with the octet rule.
C) The diatomic molecule Cl2 is an example of a polar molecule.
D) The bonds in LiF have a more covalent character than those in F2.
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the gaseous phase, which of the following diatomic molecules would be the most polar?
A) NaCl
B) CsF
C) NaF
D) LiF
E) CsCl
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for SO2.
A) tetrahedral
B) pyramidal
C) linear
D) bent
E) none of these
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following information: N2 bond energy = 941 kJ/mol
F2 bond energy = 154 kJ/mol 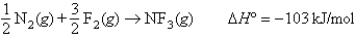 calculate the N-F bond energy.
calculate the N-F bond energy.
A) 113 kJ/mol
B) 268 kJ/mol
C) 66 kJ/mol
D) 317 kJ/mol
E) none of these
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following groups contains no ionic compounds?
A) KH, CaF2, NaNH2
B) KOH, CCl4, SF4
C) HCN, NO2, Ca(NO3) 2
D) CH2O, H2S, NBr3
E) PCl5, LiBr, Cu(OH) 2
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which case is the bond polarity incorrect?
A) ( + Cl-Br -)
B) ( + Na-S -)
C) ( + H-Br -)
D) ( + Mg-H -)
E) ( + Si-S -)
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Short Answer
For each of the following compounds: -CH4 A)Give the shape of the molecule. B)Indicate the polarity of the molecule.
Correct Answer

verified
A) tetrahe...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 61 - 80 of 135
Related Exams