Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ca is the symbol for
A) calcium.
B) carbon.
C) cobalt.
D) copper.
E) cadmium.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The primary substances of which all other things are composed are
A) molecules.
B) compounds.
C) elements.
D) electrons.
E) protons.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of an atom is equal to the number of
A) nuclei.
B) neutrons.
C) neutrons plus protons.
D) electrons plus protons.
E) protons.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Isotopes have the same atomic number but different mass numbers.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass number of an atom of potassium that has 20 neutrons?
A) 15
B) 19
C) 35
D) 39
E) 59
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT true for the atoms 13N, 14N, and 15N?
A) They all have the same mass number.
B) They are isotopes.
C) They all have the same atomic number.
D) They all have 7 protons.
E) They all have 7 electrons.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
iron
A) Ir
B) Fs
C) Fe
D) In
E) FE
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the element with the electron configuration 1s22s22p63s23p5?
A) Be
B) Cl
C) F
D) S
E) Ar
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an atom, the nucleus contains
A) an equal number of protons and electrons.
B) all the protons and neutrons.
C) all the protons and electrons.
D) only neutrons.
E) only protons.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The maximum number of electrons that may occupy the third energy level is
A) 2.
B) 8.
C) 10.
D) 18.
E) 32.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a nonmetal?
A) nitrogen
B) sodium
C) iron
D) silver
E) calcium
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives the correct numbers of protons, neutrons, and electrons in a neutral atom of 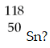
A) 118 protons, 50 neutrons, 118 electrons
B) 118 protons, 118 neutrons, 50 electrons
C) 50 protons, 68 neutrons, 50 electrons
D) 68 protons, 68 neutrons, 50 electrons
E) 50 protons, 50 neutrons, 50 electrons
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a characteristic of the modern periodic table?
A) A group is a horizontal row on the periodic table.
B) A period is a column on the periodic table.
C) The elements in each group have similar chemical properties.
D) The B groups contain the representative elements.
E) The A groups contain the transition elements.
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
Chlorine has a higher ionization energy than fluorine.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an electron-dot structure of an element, the dots are used to represent
A) all of the electrons in the atom.
B) the valence electrons.
C) the electron arrangement.
D) only the electrons that will participate in bond formation.
E) the electrons that the element will gain when it forms a compound.
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Chlorine has a higher ionization energy than aluminum.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider a neutral atom with 30 protons and 34 neutrons. The number of electrons in this atom is
A) 30.
B) 32.
C) 34.
D) 64.
E) 94.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following: 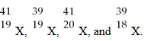 Which are isotopes of each other?
Which are isotopes of each other?
A) ![]() are isotopes of each other.
are isotopes of each other.
B) ![]() are isotopes of each other.
are isotopes of each other.
C) ![]() are isotopes of each other.
are isotopes of each other.
D) None are isotopes of each other.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Au is the symbol for
A) gold.
B) silver.
C) argon.
D) aluminum.
E) sodium.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 100
Related Exams